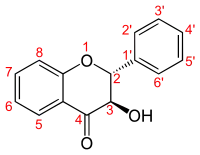
Flavanonol, numbering
The flavanonols (with two "o"s a.k.a. 3-hydroxyflavanone or 2,3-dihydroflavonol) are a class of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one (IUPAC name) backbone.
Some examples include:
- Taxifolin (or Dihydroquercetin)
- Aromadedrin (or Dihydrokaempferol)
- Engeletin (or Dihydrokaempferol-3-rhamnoside)
Metabolism
Glycosides
Glycosides (chrysandroside A and chrysandroside B) can be found in the roots of Gordonia chrysandra.[1] Xeractinol, a dihydroflavonol C-glucoside, can be isolated from the leaves of Paepalanthus argenteus var. argenteus.[2]
Dihydro-flavonol glycosides (astilbin, neoastilbin, isoastilbin, neoisoastilbin, (2R, 3R)-taxifolin-3'-O-β-D-pyranoglucoside) have been identified in the rhizome of Smilax glabra.[3]
References
- ↑ Kun Wanga; Jing Zhi Yanga; Li Zuoa; Dong Ming Zhang (January 2008). "Two new flavanonol glycosides from Gordonia chrysandra". Chinese Chemical Letters. 19 (1): 61–4. doi:10.1016/j.cclet.2007.10.033.
- ↑ Anne Lígia Dokkedal; Francisco Lavarda; Lourdes Campaner dos Santos; Wagner Vilegas (March–April 2007). "Xeractinol – a new flavanonol C-glucoside from Paepalanthus argenteus var. argenteus (Bongard) Hensold (Eriocaulaceae)". J. Braz. Chem. Soc. 18 (2): 437–439. doi:10.1590/S0103-50532007000200029. hdl:11449/36324.
- ↑ Yuan JZ, Dou DQ, Chen YJ, et al. (September 2004). "[Studies on dihydroflavonol glycosides from rhizome of Smilax glabra]". Zhongguo Zhong Yao Za Zhi (in Chinese). 29 (9): 867–70. PMID 15575206.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.