 | |
| Names | |
|---|---|
| Other names
Gallium sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.522 |
PubChem CID |
|
| |
| |
| Properties | |
| GaS• | |
| Molar mass | 101.788 g mol−1 |
| Appearance | Yellow crystals |
| Density | 3.86 g cm−3 |
| Melting point | 965 °C (1,769 °F; 1,238 K) |
| −-23.0·10−6 cm3/mol | |
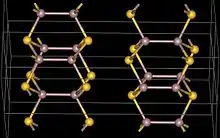
| Structure | |
| hexagonal, hP8 | |
| P63/mmc, No. 194 | |
| Related compounds | |
Related compounds |
Gallium(III) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gallium(II) sulfide, GaS, is a chemical compound of gallium and sulfur. The normal form of gallium(II) sulfide as made from the elements has a hexagonal layer structure containing Ga24+ units which have a Ga-Ga distance of 248pm.[1] This layer structure is similar to GaTe, GaSe and InSe.[1] An unusual metastable form, with a distorted wurtzite structure has been reported as being produced using MOCVD. The metal organic precursors were di-tert-butyl gallium dithiocarbamates, for example GatBu2(S2CNMe2) and this was deposited onto GaAs. The structure of the GaS produced in this way is presumably Ga2+ S2−.[2]
Single layers of gallium sulfide are dynamically stable two-dimensional semiconductors, in which the valence band has an inverted Mexican-hat shape, leading to a Lifshitz transition as the hole-doping is increased.[3]
References
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ MOCVD Growth of Gallium Sulfide Using Di-tert-butyl Gallium Dithiocarbamate Precursors: Formation of a Metastable Phase of GaS A. Keys, S G. Bott, A. R. Barron Chem. Mater., 11 (12), 3578 -3587, 1999. doi:10.1021/cm9903632
- ↑ V. Zolyomi, N. D. Drummond and V. I. Fal'ko (2013). "Band structure and optical transitions in atomic layers of hexagonal gallium chalcogenides". Phys. Rev. B. 87: 195403. arXiv:1302.6067. Bibcode:2013PhRvB..87s5403Z. doi:10.1103/PhysRevB.87.195403.