 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.203 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| La(NO 3) 3 | |
| Molar mass | 324.92 g/mol |
| Appearance | Colorless crystals |
| Odor | slight odor |
| Density | 1.3 g/cm3 |
| Melting point | 40 °C (104 °F; 313 K) |
| Boiling point | 126 °C (259 °F; 399 K) decomposes |
| 158 g/100 mL | |
| Solubility | Soluble in acetone and ethanol |
| Hazards | |
| GHS labelling: | |
 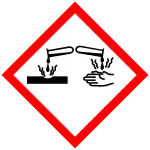  | |
| Danger | |
| H272, H315, H319, H335 | |
| P210, P273, P280, P305+P351+P338+P310, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4500 mg/kg (oral, rat)[4] |
| Related compounds | |
Other anions |
Lanthanum(III) sulfate |
Other cations |
Cerium(III) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lanthanum(III) nitrate is a water soluble salt of lanthanum with the chemical formula La(NO
3)
3. The compound decomposes at 499°C to lanthanum oxide, nitric oxide and oxygen.[3]
Uses
It is a starting chemical for the electrochemical synthesis of lanthanum permanganate and to make lanthanum oxysulfide. It is also a possible application for fluorescent display tubes.[1]
Preparation
Lanthanum nitrate is prepared by reacting lanthanum oxide with nitric acid which creates lanthanum(III) nitrate and water.
References
- 1 2 "Lanthanum(III) nitrate 99.999% trace metals". Sigma Aldrich. Retrieved 26 February 2021.
- ↑ "lanthanum nitrate". ChemSpider. Retrieved 26 February 2021.
- 1 2 Department of Chemical Engineering, The Pennsylvania State University, University Park, Pennsylvania (1996). "Influence of Pretreatment on Lanthanum Nitrate, Carbonate, and Oxide Powders". Chemistry of Materials. ACS publications. 8 (12): 2755–2768. doi:10.1021/cm9602555. Retrieved 26 February 2021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Lanthanum(III) nitrate". PubChem. Retrieved 26 February 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
