| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.348 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Rh(NO3)3 | |
| Molar mass | 288.92 g/mol |
| Appearance | Yellow solid |
| Density | 1.41 g/cm3 |
| Soluble | |
| Structure | |
| Hexagonal[3] | |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
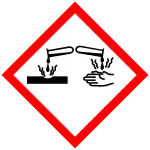
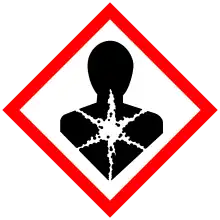
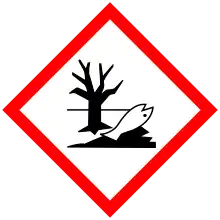
| H271, H290, H302, H314, H317, H341, H410 | |
| P201, P202, P210, P220, P221, P234, P260, P261, P264, P270, P272, P273, P280, P281, P283, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P306+P360, P308+P313, P310, P321, P330, P333+P313, P363, P370+P378, P371+P380+P375, P390, P391, P404, P405, P501 | |
| Related compounds | |
Other anions |
Rhodium(III) sulfate |
Other cations |
Cobalt(III) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Rhodium(III) nitrate is a inorganic compound, a salt of rhodium and nitric acid with the formula Rh(NO3)3. This anhydrous complex has been the subject of theoretical analysis but has not been isolated.[4] However, a dihydrate and an aqueous solution are known with similar stoichiometry; they contain various hexacoordinated rhodium(III) aqua and nitrate complexes.[3] A number of other rhodium nitrates have been characterized by X-ray crystallography: Rb4[trans-[Rh(H2O)2(NO3)4][Rh(NO3)6][4] and Cs2[-[Rh(NO3)5].[5] Rhodium nitrates are of interest because nuclear wastes, which contain rhodium, are recycled by dissolution in nitric acid.[6]
Uses
Rhodium(III) nitrate is used as a precursor to synthesize rhodium.[7]
References
- ↑ "Rhodium nitrate". PubChem. Retrieved 12 March 2021.
- ↑ "Rhodium nitrate". American Elements. Retrieved 12 March 2021.
- 1 2 G. Bongiovanni; R. Caminiti; D. Atzei; P. Cucca; A. Anedda (1986). "Structure of rhodium(III) nitrate aqueous solutions. An investigation by x-ray diffraction and Raman spectroscopy". The Journal of Physical Chemistry. ACS Publications. 90 (2): 238–243. doi:10.1021/j100274a007. Retrieved 12 March 2021.
- 1 2 Vasilchenko D.; Vasilchenko D.; Vorob'eva S.; Tkachev S.; Baidina I.; Belyaev A.;Korenev S.; Solovyov L.;Vasiliev, A. (2016). "Rhodium(III) Speciation in Concentrated Nitric Acid Solutions". European Journal of Inorganic Chemistry. 2016 (23): 3822 - 3828. doi:10.1002/ejic.201600523.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Vasilchenko, Danila; Vorobieva, Sofia; Baidina, Iraida; Piryazev, Dmitry; Tsipis, Athanassios; Korenev, Sergey (2018). "Structure and properties of a rhodium(III) pentanitrato complex embracing uni- and bidentate nitrato ligands". Polyhedron. 147: 69–74. doi:10.1016/j.poly.2018.03.017. S2CID 104064801.
- ↑ Samuels, Alex C.; Boele, Cherilynn A.; Bennett, Kevin T.; Clark, Sue B.; Wall, Nathalie A.; Clark, Aurora E. (2014). "Integrated Computational and Experimental Protocol for Understanding Rh(III) Speciation in Hydrochloric and Nitric Acid Solutions". Inorganic Chemistry. 53 (23): 12315–12322. doi:10.1021/ic501408r. PMID 25390284.
- ↑ "Rhodium(III) nitrate hydrate". Sigma Aldrich. Retrieved 12 March 2021.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.