| left-right determination factor 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | LEFTY1 | ||||||
| Alt. symbols | LEFTB | ||||||
| NCBI gene | 10637 | ||||||
| HGNC | 6552 | ||||||
| OMIM | 603037 | ||||||
| RefSeq | NM_020997 | ||||||
| UniProt | O75610 | ||||||
| Other data | |||||||
| Locus | Chr. 1 q42.1 | ||||||
| |||||||
| left-right determination factor 2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | LEFTY2 | ||||||
| Alt. symbols | TGFB4, EBAF | ||||||
| NCBI gene | 7044 | ||||||
| HGNC | 3122 | ||||||
| OMIM | 601877 | ||||||
| RefSeq | NM_003240 | ||||||
| UniProt | O00292 | ||||||
| Other data | |||||||
| Locus | Chr. 1 q42.1 | ||||||
| |||||||
Lefty (left-right determination factors) are a class of proteins that are closely related members of the TGF-beta superfamily of growth factors. These proteins are secreted and play a role in left-right asymmetry determination of organ systems during development.[1] Mutations of the genes encoding these proteins have been associated with left-right axis malformations, particularly in the heart and lungs.[2]
History
Lefty, a divergent member of the transforming growth factor-β (TGF beta) superfamily of proteins, was originally discovered in the Hamada lab at the Osaka University using deletion screening of cDNA libraries in P19 embryonic carcinoma cells to find clones that did not differentiate when induced to differentiate using retinoic acid. From these screens, researchers found one gene that was a tentative member of the TGF-beta superfamily that was predominantly expressed on the left side the embryo and aptly named it lefty.[3] Like other members of the TGF-beta superfamily, lefty is synthesized as a preproprotein, meaning that the protein is proteolytically cleaved and excreted to produce the active form of the protein. However, lefty has only 20-25% sequence similarity with other members of the TGF-beta superfamily. Lefty is conserved in all vertebrates and many species have more than one homologue. Humans and mice, for instance have two homologues, Lefty 1 and Lefty 2, whose differential expression leads to distinct purposes while the mechanism of action is conserved.[4]
Function
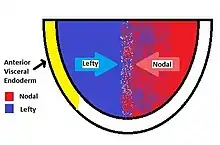
Lefty proteins function as an antagonist of the Nodal Signaling pathway. Nodal is another signaling protein which is responsible for gastrulation, left-right patterning and induction of the primitive node. As NODAL protein diffuse through an embryo, it triggers Nodal Signaling within tissues with the required receptors and coreceptors. Activated nodal signaling leads to the transcription of the lefty gene. The protein is then expressed, proteolytically cleaved, and finally secreted. Secreted lefty binds to EGF-CFC proteins like one-eyed pinhead in zebrafish keeping the essential cofactor from associating with NODAL/ Activin-like receptor complex. This will effectually block Nodal Signaling. During induction of the primitive streak, lefty confines Nodal activity to the posterior end of the embryo, establishing a posterior signaling center and inducing the formation of the primitive streak and mesoderm.[5] (See Nodal Signaling or TGF beta signaling pathway for more information on the nodal signaling pathway.)[6]
There are many differences between the left and right sides, including heart and lung positioning. Mutations in these genes cause incorrect positioning of these organs (e.g., situs inversus), or in the case of constitutively inactive lefty, the embryo becomes entirely mesoderm and fails to pattern or develop. During vertebrate development, lefty proteins regulate left-right asymmetry by controlling the spatiotemporal influence of the NODAL protein. Lefty1 in the ventral midline prevents the Cerberus (paracrine factor or "Caronte") signal from passing to the right side of the embryo.[1] This spatiotemporal control is achieved by using two sources of excreted lefty. While lefty is produced in response to activated nodal signaling, it is also produced and secreted in the anterior visceral endoderm (AVE). The balance of lefty from the AVE and from Nodal Signaling results in the patterning of the embryo and left-right asymmetry.[7]
Clinical significance
Proper functioning of Lefty is crucial to the proper development of the heart, lungs, spleen, and liver. Mutations in Lefty, called Lefty-A, are associated with left-right patterning defects. This mutation may cause congenital heart defects due to malformation, interrupted inferior vena cava, and lack of lung asymmetry (left pulmonary isomerism).[5] Lefty2 may play a role in endometrial bleeding.[8][9]
Lefty-1
Lefty-1 is a regulatory gene that plays a vital role in the determination of the left-right internal asymmetry observed in mammals. The lefty-1 protein works in tandem with two other genes: lefty-2 and nodal. As the primitive node migrates towards the cranial end of the embryo during development, its cilia preferentially sling lefty-2 and nodal towards the left side of the embryo.[10] These two genes encode for “leftness”, and initiate the formation of the heart, spleen, and other internal organs that are found on the left side in a typical human being. Lefty-1 protein can be viewed as a barrier between the left and right portions of the embryo that prevents the diffusion of lefty-2 and nodal to the right side. This ensures that the left-determining molecules are confined to their correct developmental domain. A variety of defects were observed in mice that had lefty-1 deleted, including left pulmonary isomerism, situs inversus, and atrial septal defect [2]. The high incidence of left pulmonary isomerism in the knockout mice indicates that lefty-1 itself is not involved in encoding for leftness, but simply ensures the correct compartmentation of the left-determining molecules. In the absence of the lefty-1 barrier, lefty-2 and nodal are free to diffuse to the right side and initiate the development of a left lung that was meant to be limited to the left side of the thoracic cavity.
References
- 1 2 Hamada H, Meno C, Watanabe D, Saijoh Y (February 2002). "Establishment of vertebrate left-right asymmetry". Nat. Rev. Genet. 3 (2): 103–13. doi:10.1038/nrg732. PMID 11836504. S2CID 20557143.
- ↑ Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H (August 1998). "lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal". Cell. 94 (3): 287–97. doi:10.1016/S0092-8674(00)81472-5. PMID 9708731. S2CID 5666974.
- ↑ Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H (May 1996). "Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos". Nature. 381 (6578): 151–5. Bibcode:1996Natur.381..151M. doi:10.1038/381151a0. PMID 8610011. S2CID 4345275.
- ↑ Kosaki K, Bassi MT, Kosaki R, Lewin M, Belmont J, Schauer G, Casey B (March 1999). "Characterization and mutation analysis of human LEFTY A and LEFTY B, homologues of murine genes implicated in left-right axis development". Am. J. Hum. Genet. 64 (3): 712–21. doi:10.1086/302289. PMC 1377788. PMID 10053005.
- 1 2 Carlson, Bruce M. "Formation of Germ Layers and Early Derivatives." Human Embryology and Developmental Biology. Philadelphia, Pennsylvania: Mosby/Elsevier, 2009. 91-95. Print.
- ↑ Schier AF (November 2009). "Nodal Morphogens". Cold Spring Harb Perspect Biol. 1 (5): a003459. doi:10.1101/cshperspect.a003459. PMC 2773646. PMID 20066122.
- ↑ Takaoka K, Yamamoto M, Hamada H (August 2007). "Origin of body axes in the mouse embryo". Curr. Opin. Genet. Dev. 17 (4): 344–50. doi:10.1016/j.gde.2007.06.001. PMID 17646095.
- ↑ Kothapalli R, Buyuksal I, Wu SQ, Chegini N, Tabibzadeh S (May 1997). "Detection of ebaf, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding". J. Clin. Invest. 99 (10): 2342–50. doi:10.1172/JCI119415. PMC 508072. PMID 9153275.
- ↑ Tabibzadeh S (2005). "Role of EBAF/Lefty in implantation and uterine bleeding". Ernst Schering Res. Found. Workshop. Ernst Schering Research Foundation Workshop. 52 (52): 159–89. doi:10.1007/3-540-27147-3_8. ISBN 978-3-540-23089-2. PMID 15704472.
- ↑ Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H (February 2010). "Planar polarization of node cells determines the rotational axis of node cilia". Nature Cell Biology. 12 (2): 170–6. doi:10.1038/ncb2020. PMID 20098415. S2CID 6379844.
Further reading
- Carlson BM (2014). "Formation of germ layers and early derivatives.". Human Embryology and Developmental Biology. Philadelphia, Pennsylvania: Mosby/Elsevier. pp. 75–91. ISBN 978-0-323-08279-2.
- Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H (April 2002). "Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion". Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 7 (4): 401–12. doi:10.1046/j.1365-2443.2002.00528.x. PMID 11952836. S2CID 19320756.