 | |
| Names | |
|---|---|
| Other names
Manganese(II) stearate, manganese distearate, manganese(2+) dioctadecanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.110 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 36H 70MnO 4 | |
| Molar mass | 621.89 |
| Appearance | Pale pink powder |
| Density | g/cm3 |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| insoluble | |
| Hazards | |
| GHS labelling: | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| Flash point | 162.4 °C (324.3 °F; 435.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
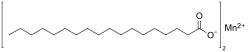
Manganese stearate is a metal-organic compound, a salt of manganese and stearic acid with the chemical formula C
36H
70MnO
4.[1][2] The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[3]
Synthesis
Manganese stearate is synthesized by the reaction of stearic acid with sodium hydroxide, followed by reacting with manganese chloride.[4]
Also, the reaction of manganese(II) acetate with stearic acid.[5]
Physical properties
The compound forms pale pink powder.[6]
Insoluble in water.[6]
Uses
The compound is used in organic synthesis reactions.[6]
Also as an oxidant additive for oxo-biodegradable polymers (for example, high-density polyethylene).[7]
References
- ↑ "Manganese Stearate". American Elements. Retrieved 6 March 2023.
- ↑ "NCATS Inxight Drugs — MANGANESE STEARATE". National Center for Advancing Translational Sciences. Retrieved 6 March 2023.
- ↑ "CAS 3353-05-7 Manganese Stearate - Alfa Chemistry". alfa-chemistry.com. Retrieved 6 March 2023.
- ↑ Aras, Neny Rasnyanti M.; Arcana, I Made (2015). "Synthesis of manganese stearate for high density polyethylene (HDPE) and its biodegradation". AIP Conference Proceedings. 1677 (1): 070024. Bibcode:2015AIPC.1677g0024A. doi:10.1063/1.4930728. Retrieved 6 March 2023.
- ↑ "manganese stearate". chemsrc.com. Retrieved 6 March 2023.
- 1 2 3 "Manganese Stearate | CAS 3353-05-7". Santa Cruz Biotechnology. Retrieved 6 March 2023.
- ↑ Roy, Prasun Kumar; Singh, Priyanka; Kumar, Devendra; Rajagopal, Chitra (2010). "Manganese stearate initiated photo-oxidative and thermo-oxidative degradation of LDPE, LLDPE and their blends". Journal of Applied Polymer Science: NA. doi:10.1002/app.31252.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.