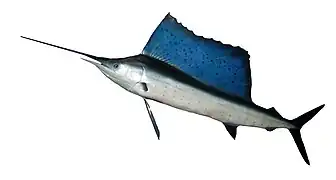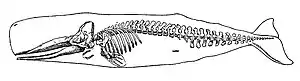
Marine vertebrates are vertebrates that live in marine environments. These are the marine fish and the marine tetrapods (primarily seabirds, marine reptiles, and marine mammals). Vertebrates are a subphylum of chordates that have a vertebral column (backbone). The vertebral column provides the central support structure for an internal skeleton. The internal skeleton gives shape, support, and protection to the body and can provide a means of anchoring fins or limbs to the body. The vertebral column also serves to house and protect the spinal cord that lies within the column.
Marine vertebrates can be divided into two groups, marine fish and marine tetrapods.
Marine fish
| Part of a series of overviews on |
| Marine life |
|---|
 |
Fish fall into two main groups: fish with bony internal skeletons and fish with cartilaginous internal skeletons. Fish anatomy and physiology generally includes a two-chambered heart, eyes adapted to seeing underwater, and a skin protected by scales and mucous. They typically breathe by extracting oxygen from water through gills. Fish use fins to propel and stabilise themselves in the water. Over 33,000 species of fish have been described as of 2017,[1] of which about 20,000 are marine fish.[2]
Jawless fish
Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of jawless fish.[3] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[4] only 18 species of lampreys are actually parasitic.[5] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[6] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[7] Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[8] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of craniata.[9]
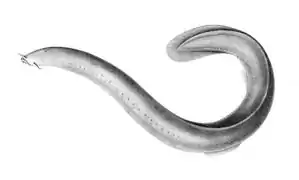
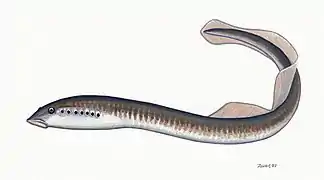 Lampreys are often parasitic and have a toothed, funnel-like sucking mouth
Lampreys are often parasitic and have a toothed, funnel-like sucking mouth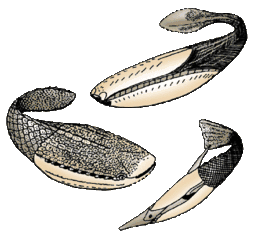 The extinct Pteraspidomorphi, ancestral to jawed vertebrates
The extinct Pteraspidomorphi, ancestral to jawed vertebrates
Pteraspidomorphi is an extinct class of early jawless fish ancestral to jawed vertebrates. The few characteristics they share with the latter are now considered as primitive for all vertebrates.
Cartilaginous fish
Cartilaginous fish, such as sharks and rays, have jaws and skeletons made of cartilage rather than bone. Megalodon is an extinct species of shark that lived about 28 to 1.5 Ma. It looked much like a stocky version of the great white shark, but was much larger with fossil lengths reaching 20.3 metres (67 ft).[10] Found in all oceans[11] it was one of the largest and most powerful predators in vertebrate history,[10] and probably had a profound impact on marine life.[12] The Greenland shark has the longest known lifespan of all vertebrates, about 400 years.[13]
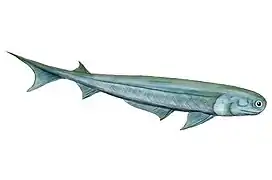 Cartilaginous fishes may have evolved from spiny sharks
Cartilaginous fishes may have evolved from spiny sharks

 Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[15]
Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[15]
 The extinct megalodon resembled a giant great white shark
The extinct megalodon resembled a giant great white shark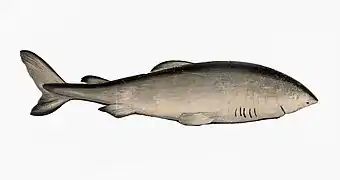 The Greenland shark lives longer than any other vertebrate
The Greenland shark lives longer than any other vertebrate.jpg.webp)
Bony fish
Bony fish have jaws and skeletons made of bone rather than cartilage. About 90% of the world's fish species are bony fish. Bony fish also have hard, bony plates called operculum which help them respire and protect their gills, and they often possess a swim bladder which they use for better control of their buoyancy.
Bony fish can be further divided into those with lobe fins and those with ray fins. Lobe fins have the form of fleshy lobes supported by bony stalks which extend from the body.[16] Lobe fins evolved into the legs of the first tetrapod land vertebrates, so by extension an early ancestor of humans was a lobe-finned fish. Apart from the coelacanths and the lungfishes, lobe-finned fishes are now extinct. The rest of the modern fish have ray fins. These are made of webs of skin supported by bony or horny spines (rays) which can be erected to control the fin stiffness.
Marine tetrapods

A tetrapod (Greek for four feet) is a vertebrate with limbs (feet). Tetrapods evolved from ancient lobe-finned fishes about 400 million years ago during the Devonian Period when their earliest ancestors emerged from the sea and adapted to living on land.[17] This change from a body plan for breathing and navigating in gravity-neutral water to a body plan with mechanisms enabling the animal to breath in air without dehydrating and move on land is one of the most profound evolutionary changes known.[18][19] Tetrapods can be divided into four classes: amphibians, reptiles, birds and mammals.
Marine tetrapods are tetrapods that returned from land back to the sea again. The first returns to the ocean may have occurred as early as the Carboniferous Period[20] whereas other returns occurred as recently as the Cenozoic, as in cetaceans, pinnipeds,[21] and several modern amphibians.[22]
Amphibians
Amphibians (Greek for both kinds of life) live part of their life in water and part on land. They mostly require fresh water to reproduce. A few inhabit brackish water, but there are no true marine amphibians.[23] There have been reports, however, of amphibians invading marine waters, such as a Black Sea invasion by the natural hybrid Pelophylax esculentus reported in 2010.[24]
Reptiles
Reptiles (Late Latin for creeping or crawling) do not have an aquatic larval stage, and in this way are unlike amphibians. Most reptiles are oviparous, although several species of squamates are viviparous, as were some extinct aquatic clades[25] — the fetus develops within the mother, contained in a placenta rather than an eggshell. As amniotes, reptile eggs are surrounded by membranes for protection and transport, which adapt them to reproduction on dry land. Many of the viviparous species feed their fetuses through various forms of placenta analogous to those of mammals, with some providing initial care for their hatchlings.
Some reptiles are more closely related to birds than other reptiles, and many scientists prefer to make Reptilia a monophyletic group which includes the birds.[26][27][28][29] Extant non-avian reptiles which inhabit or frequent the sea include sea turtles, sea snakes, terrapins, the marine iguana, and the saltwater crocodile. Currently, of the approximately 12,000 extant reptile species and sub-species, only about 100 of are classed as marine reptiles.[30]
Except for some sea snakes, most extant marine reptiles are oviparous and need to return to land to lay their eggs. Apart from sea turtles, the species usually spend most of their lives on or near land rather than in the ocean. Sea snakes generally prefer shallow waters nearby land, around islands, especially waters that are somewhat sheltered, as well as near estuaries.[31][32] Unlike land snakes, sea snakes have evolved flattened tails which help them swim.[33]

.jpg.webp)
.jpg.webp)
 Marine snakes have flattened tails
Marine snakes have flattened tails The ancient Ichthyosaurus communis independently evolved flippers similar to dolphins
The ancient Ichthyosaurus communis independently evolved flippers similar to dolphins
Some extinct marine reptiles, such as ichthyosaurs, evolved to be viviparous and had no requirement to return to land. Ichthyosaurs resembled dolphins. They first appeared about 245 million years ago and disappeared about 90 million years ago. The terrestrial ancestor of the ichthyosaur had no features already on its back or tail that might have helped along the evolutionary process. Yet the ichthyosaur developed a dorsal and tail fin which improved its ability to swim.[34] The biologist Stephen Jay Gould said the ichthyosaur was his favourite example of convergent evolution.[35] The earliest marine reptiles arose in the Permian. During the Mesozoic many groups of reptiles became adapted to life in the seas, including ichthyosaurs, plesiosaurs, mosasaurs, nothosaurs, placodonts, sea turtles, thalattosaurs and thalattosuchians. Marine reptiles were less numerous after mass extinction at the end of the Cretaceous.
Birds
Marine birds are adapted to life within the marine environment. They are often called seabirds. While marine birds vary greatly in lifestyle, behaviour and physiology, they often exhibit striking convergent evolution, as the same environmental problems and feeding niches have resulted in similar adaptations. Examples include albatross, penguins, gannets, and auks.
In general, marine birds live longer, breed later and have fewer young than terrestrial birds do, but they invest a great deal of time in their young. Most species nest in colonies, which can vary in size from a few dozen birds to millions. Many species are famous for undertaking long annual migrations, crossing the equator or circumnavigating the Earth in some cases. They feed both at the ocean's surface and below it, and even feed on each other. Marine birds can be highly pelagic, coastal, or in some cases spend a part of the year away from the sea entirely. Some marine birds plummet from heights, plunging through the water leaving vapour-like trails, similar to that of fighter planes.[36] Gannets plunge into the water at up to 100 kilometres per hour (60 mph). They have air sacs under their skin in their face and chest which act like bubble-wrap, cushioning the impact with the water.
 European herring gull attack herring schools from above
European herring gull attack herring schools from above Gentoo penguin swimming underwater
Gentoo penguin swimming underwater Gannets "divebomb" at high speed
Gannets "divebomb" at high speed Albatrosses range over huge areas of ocean and regularly circle the globe.
Albatrosses range over huge areas of ocean and regularly circle the globe.
The first marine birds evolved in the Cretaceous period, and modern marine bird families emerged in the Paleogene.
Mammals

Mammals (from Latin for breast) are characterised by the presence of mammary glands which in females produce milk for feeding (nursing) their young. There are about 130 living and recently extinct marine mammal species such as seals, dolphins, whales, manatees, sea otters and polar bears.[37] They do not represent a distinct taxon or systematic grouping, but are instead unified by their reliance on the marine environment for feeding. Both cetaceans and sirenians are fully aquatic and therefore are obligate water dwellers. Seals and sea-lions are semiaquatic; they spend the majority of their time in the water, but need to return to land for important activities such as mating, breeding and molting. In contrast, both otters and the polar bear are much less adapted to aquatic living. Their diet varies considerably as well: some may eat zooplankton; others may eat fish, squid, shellfish, and sea-grass; and a few may eat other mammals.
In a process of convergent evolution, marine mammals such as dolphins and whales redeveloped their body plan to parallel the streamlined fusiform body plan of pelagic fish. Front legs became flippers and back legs disappeared, a dorsal fin reappeared and the tail morphed into a powerful horizontal fluke. This body plan is an adaptation to being an active predator in a high drag environment. A parallel convergence occurred with the now extinct ichthyosaur.[38]
 Endangered blue whale, largest animal ever[39]
Endangered blue whale, largest animal ever[39] Humpback whale straining krill
Humpback whale straining krill

 Walrus coming up for air
Walrus coming up for air Battling sea elephants
Battling sea elephants.jpg.webp)
See also
References
- ↑ "FishBase: A Global Information System on Fishes". FishBase. Retrieved 17 January 2017.
- ↑ "How Many Fish In The Sea? Census Of Marine Life Launches First Report". Science Daily. Retrieved 17 January 2017.
- ↑ Docker, Margaret F. (2006). "Bill Beamish's Contributions to Lamprey Research and Recent Advances in the Field". Guelph Ichthyology Reviews. 7. doi:10.1111/j.1095-8649.2006.00968.x (inactive 1 August 2023).
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - ↑ Hardisty, M. W.; Potter, I. C. (1971). Hardisty, M. W.; Potter, I. C. (eds.). The Biology of Lampreys (1st ed.). Academic Press. ISBN 978-0-123-24801-5.
- ↑ Gill, Howard S.; Renaud, Claude B.; Chapleau, François; Mayden, Richard L.; Potter, Ian C.; Douglas, M. E. (2003). "Phylogeny of Living Parasitic Lampreys (Petromyzontiformes) Based on Morphological Data". Copeia. 2003 (4): 687–703. doi:10.1643/IA02-085.1. S2CID 85969032.
- ↑ "Myxini". University of California Museum of Paleontology. Archived from the original on 15 December 2017. Retrieved 17 January 2017.
- ↑ Greena, Stephen A.; Bronner, Marianne E. (2014). "The Lamprey: A jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits". Differentiation. 87 (1–2): 44–51. doi:10.1016/j.diff.2014.02.001. PMC 3995830. PMID 24560767.
- ↑ Stock, D.; Whitt, G.S. (7 August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfish form a natural group". Science. 257 (5071): 787–9. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- ↑ Nicholls, H. (10 September 2009). "Mouth to Mouth". Nature. 461 (7261): 164–166. doi:10.1038/461164a. PMID 19741680. S2CID 35838285.
- 1 2 Wroe, S.; Huber, D. R.; Lowry, M.; McHenry, C.; Moreno, K.; Clausen, P.; Ferrara, T. L.; Cunningham, E.; Dean, M. N.; Summers, A. P. (2008). "Three-dimensional computer analysis of white shark jaw mechanics: how hard can a great white bite?" (PDF). Journal of Zoology. 276 (4): 336–342. doi:10.1111/j.1469-7998.2008.00494.x.
- ↑ Pimiento, Catalina; Dana J. Ehret; Bruce J. MacFadden; Gordon Hubbell (10 May 2010). Stepanova, Anna (ed.). "Ancient Nursery Area for the Extinct Giant Shark Megalodon from the Miocene of Panama". PLOS ONE. 5 (5): e10552. Bibcode:2010PLoSO...510552P. doi:10.1371/journal.pone.0010552. PMC 2866656. PMID 20479893.
- ↑ Lambert, Olivier; Bianucci, Giovanni; Post, Klaas; de Muizon, Christian; Salas-Gismondi, Rodolfo; Urbina, Mario; Reumer, Jelle (1 July 2010). "The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru". Nature. 466 (7302): 105–108. Bibcode:2010Natur.466..105L. doi:10.1038/nature09067. PMID 20596020. S2CID 4369352.
- ↑ Nielsen, Julius; Hedeholm, Rasmus B.; Heinemeier, Jan; Bushnell, Peter G.; Christiansen, Jørgen S.; Olsen, Jesper; Ramsey, Christopher Bronk; Brill, Richard W.; Simon, Malene; Steffensen, Kirstine F.; Steffensen, John F. (2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–4. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. hdl:2022/26597. PMID 27516602. S2CID 206647043.
- Enrico de Lazaro (12 August 2016). "Greenland Sharks are Longest-Lived Vertebrates on Earth, Marine Biologists Say". Science News.
- ↑ Marshall, A.; Bennett, M.B.; Kodja, G.; Hinojosa-Alvarez, S.; Galvan-Magana, F.; Harding, M.; Stevens, G. & Kashiwagi, T. (2011). "Manta birostris". IUCN Red List of Threatened Species. 2011: e.T198921A9108067. doi:10.2305/IUCN.UK.2011-2.RLTS.T198921A9108067.en.
- ↑ Black, Richard (11 June 2007). "Sawfish protection acquires teeth". BBC News.
- ↑ Clack, J. A. (2002) Gaining Ground. Indiana University
- ↑ Narkiewicz, Katarzyna; Narkiewicz, Marek (January 2015). "The age of the oldest tetrapod tracks from Zachełmie, Poland". Lethaia. 48 (1): 10–12. doi:10.1111/let.12083. ISSN 0024-1164.
- ↑ Long JA, Gordon MS (September–October 2004). "The greatest step in vertebrate history: a paleobiological review of the fish-tetrapod transition". Physiol. Biochem. Zool. 77 (5): 700–19. doi:10.1086/425183. PMID 15547790. S2CID 1260442. as PDF
- ↑ Shubin, N. (2008). Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body. New York: Pantheon Books. ISBN 978-0-375-42447-2.
- ↑ Laurin, M. (2010). How Vertebrates Left the Water. Berkeley, California, USA.: University of California Press. ISBN 978-0-520-26647-6.
- ↑ Canoville, Aurore; Laurin, Michel (2010). "Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on paleobiological inferences". Biological Journal of the Linnean Society. 100 (2): 384–406. doi:10.1111/j.1095-8312.2010.01431.x.
- ↑ Laurin, Michel; Canoville, Aurore; Quilhac, Alexandra (2009). "Use of paleontological and molecular data in supertrees for comparative studies: the example of lissamphibian femoral microanatomy". Journal of Anatomy. 215 (2): 110–123. doi:10.1111/j.1469-7580.2009.01104.x. PMC 2740958. PMID 19508493.
- ↑ Hopkins Gareth R.; Brodie Edmund D. Jr (2015). "Occurrence of Amphibians in Saline Habitats: A Review and Evolutionary Perspective". Herpetological Monographs. 29 (1): 1–27. doi:10.1655/HERPMONOGRAPHS-D-14-00006. S2CID 83659304.
- ↑ Natchev, Nikolay; Tzankov, Nikolay; Geme, Richard (2011). "Green frog invasion in the Black Sea: habitat ecology of the Pelophylax esculentus complex (Anura, Amphibia) population in the region of Shablenska Tuzla lagoon in Bulgaria" (PDF). Herpetology Notes. 4: 347–351. Trematosaurs were also possibly marine.
- ↑ Sander, P. Martin (2012). "Reproduction in early amniotes". Science. 337 (6096): 806–808. Bibcode:2012Sci...337..806S. doi:10.1126/science.1224301. PMID 22904001. S2CID 7041966.
- ↑ Modesto, S.P.; Anderson, J.S. (2004). "The phylogenetic definition of Reptilia". Systematic Biology. 53 (5): 815–821. doi:10.1080/10635150490503026. PMID 15545258.
- ↑ Gauthier, J.A.; Kluge, A.G.; Rowe, T. (1988). "The early evolution of the Amniota". In Benton, M.J. (ed.). The Phylogeny and Classification of the Tetrapods. Vol. 1. Oxford: Clarendon Press. pp. 103–155. ISBN 978-0-19-857705-8.
- ↑ Laurin, M.; Reisz, R. R. (1995). "A reevaluation of early amniote phylogeny". Zoological Journal of the Linnean Society. 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x.
- ↑ Modesto, S.P. (1999). "Observations of the structure of the Early Permian reptile Stereosternum tumidum Cope". Palaeontologia Africana. 35: 7–19.
- ↑ Rasmussen, Arne Redsted; Murphy, John C.; Ompi, Medy; Gibbons, J. Whitfield; Uetz, Peter (8 November 2011). "Marine Reptiles". PLOS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
- ↑ Stidworthy J. 1974. Snakes of the World. Grosset & Dunlap Inc. 160 pp. ISBN 0-448-11856-4.
- ↑ Sea snakes at Food and Agriculture Organization of the United Nations. Accessed 7 August 2007.
- ↑ Rasmussen, A.R.; Murphy, J.C.; Ompi, M.; Gibbons, J.W.; Uetz, P. (2011). "Marine reptiles". PLOS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
- ↑ Martill D.M. (1993). "Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany". Kaupia - Darmstädter Beiträge zur Naturgeschichte, 2 : 77-97.
- ↑ Gould, Stephen Jay (1993) "Bent Out of Shape" in Eight Little Piggies: Reflections in Natural History. Norton, 179–94. ISBN 9780393311396.
- ↑ "Sardine Run Shark Feeding Frenzy Phenomenon in Africa". Archived from the original on 2 December 2008.
- ↑ "The Society for Marine Mammalogy's Taxonomy Committee List of Species and subspecies". Society for Marine Mammalogy. October 2015. Archived from the original on 6 January 2015. Retrieved 23 November 2015.
- ↑ Romer A. S. and Parsons T. S. (1986) The Vertebrate Body, page 96, Sanders College Publishing. ISBN 0030584469.
- ↑ "Blue whale". World Wide Fund For Nature. Retrieved 15 August 2016.
- ↑ Marino, Lori (2004). "Cetacean Brain Evolution: Multiplication Generates Complexity" (PDF). International Society for Comparative Psychology (17): 1–16. Archived from the original (PDF) on 16 September 2018. Retrieved 18 May 2019.

.jpg.webp)