 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ovral, Opill, others |
| Other names | dl-Norgestrel; DL-Norgestrel; (±)-Norgestrel; WY-3707; SH-70850; SH-850; FH 122-A; rac-13-Ethyl-17α-ethynyl-19-nortestosterone; rac-13-Ethyl-17α-ethynylestr-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a602008 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.026.758 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norgestrel is a progestin which is used in birth control pills sold under the brand name Ovral in combination with the estrogen ethinylestradiol and Opill by itself. It is also used in menopausal hormone therapy.[2][3][4][5][6] It is taken by mouth.[4][5]
Side effects of norgestrel include menstrual irregularities, headaches, nausea, and breast tenderness.[7] The most common side effects of the progestin-only Opill include irregular bleeding, headaches, dizziness, nausea, increased appetite, abdominal pain, cramps, or bloating.[1] Norgestrel is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[5] It has weak androgenic activity and no other important hormonal activity.[5]
Norgestrel was patented in 1961 and came into medical use, specifically in birth control pills, in 1966.[8][9][10] It was subsequently introduced for use in menopausal hormone therapy as well.[6] Norgestrel is sometimes referred to as a "second-generation" progestin.[11] It is marketed widely throughout the world.[6][3] Norgestrel is available as a generic medication.[12] In 2020, the version with ethinylestradiol was the 316th most commonly prescribed medication in the United States, with more than 900 thousand prescriptions.[13][14] In July 2023, the US Food and Drug Administration (FDA) approved norgestrel for over-the-counter sale.[1] The FDA granted the approval to Laboratoire HRA Pharma which was acquired by Perrigo Company plc.[1]
Medical uses
Norgestrel is used in combination with ethinylestradiol or quinestrol in combined birth control pills, alone in progestogen-only birth control pills, and in combination with estradiol or conjugated estrogens in menopausal hormone therapy.[6] It has also been used as an emergency contraceptive in the Yuzpe regimen.[15]
Side effects
Pharmacology
Pharmacodynamics
Norgestrel is a progestogen, or an agonist of the progesterone receptor.[5] The biological activity of norgestrel lies in the levo enantiomer, levonorgestrel, whereas the dextro isomer is inactive.[5] As such, norgestrel is identical in its hormonal activity to levonorgestrel except that it is half as potent by weight.[5] Levonorgestrel, and by extension norgestrel, have some androgenic activity, but no estrogenic, antimineralocorticoid, or glucocorticoid activity.[5]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Levonorgestrel | 150–162 | 34a, 45 | 0 | 1–8 | 17–75 | 50 | 0 |
| 5α-Dihydrolevonorgestrel | 50 | 38a | 0 | ? | ? | ? | ? |
| 3α,5α-Tetrahydrolevonorgestrel | ? | ? | 0.4 | ? | ? | ? | ? |
| 3β,5α-Tetrahydrolevonorgestrel | ? | ? | 2.4 | ? | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone (a = mibolerone) for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | |||||||
The ovulation-inhibiting dose of norgestrel appears to be greater than 75 μg/day, as ovulation occurred in 50 to 75% of cycles with this dosage of norgestrel in studies.[16] The ovulation-inhibiting dosage of levonorgestrel, which is twice as potent as norgestrel, is approximately 50 to 60 μg/day.[5][17][16] One review lists the ovulation-inhibiting dose of norgestrel as 100 μg/day.[18] The endometrial transformation dose of norgestrel is listed as 12 mg per cycle and the menstrual delay test dose of norgestrel is listed as 0.5 to 2 mg/day.[18][19]
Pharmacokinetics
The pharmacokinetics of norgestrel have been reviewed.[20]
Chemistry
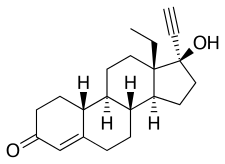
Norgestrel, also known as rac-13-ethyl-17α-ethynyl-19-nortestosterone or as rac-13-ethyl-17α-ethynylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[2][3] It is a racemic mixture of stereoisomers dextronorgestrel (the C13α isomer; l-norgestrel, L-norgestrel, or (+)-norgestrel) and levonorgestrel (the C13β isomer; d-norgestrel, D-norgestrel, or (–)-norgestrel), the former of which is inactive (making norgestrel exactly half as potent as levonorgestrel).[21][22] Norgestrel is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[23]
Synthesis
Chemical syntheses of norgestrel have been published.[20]
History
Norgestrel was first introduced, as a birth control pill in combination with ethinylestradiol, under the brand name Eugynon in Germany in 1966.[8][9] It was subsequently marketed as a combined birth control pill with ethinylestradiol in the United States under the brand name Ovral in 1968, and was marketed in many other countries as well.[24][25][6]
The contraceptive efficacy of norgestrel was established in the US with the original approval for prescription use in 1973.[1]
Society and culture
Generic names
Norgestrel is the generic name of the drug and its INN, USAN, USP, BAN, DCF, DCIT, and JAN.[2][3][4][6] It is also known as dl-norgestrel, DL-norgestrel, or (±)-norgestrel.[2][3][4][6]
Brand names
Norgestrel has been marketed under a variety of brand names including Cyclacur, Cryselle, Cyclo-Progynova, Duoluton, Elinest, Eugynon, Microgynon, Lo/Ovral, Low-Ogestrel, Logynon, Microlut, Minicon, Nordette, Neogest, Ogestrel, Ovral, Ovran, Ovranette, Ovrette, Planovar, Prempak, Progyluton, and Trinordiol among others.[2][3][6][24]
See also
References
- 1 2 3 4 5 "FDA Approves First Nonprescription Daily Oral Contraceptive". U.S. Food and Drug Administration (FDA) (Press release). 2023-07-13. Retrieved 2023-07-13.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 751–. ISBN 978-3-88763-075-1.
- 1 2 3 4 Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 202–. ISBN 978-94-011-4439-1. Archived from the original on 10 January 2023. Retrieved 10 March 2018.
- 1 2 3 4 5 6 7 8 9 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324. Archived (PDF) from the original on 2016-08-22. Retrieved 2018-03-10.
- 1 2 3 4 5 6 7 8 "Norgestrel - brand name list from". Drugs.com. Archived from the original on 2021-01-09. Retrieved 2022-09-17.
- ↑ Research, Center for Drug Evaluation and (2023-07-14). "Opill (0.075mg Oral Norgestrel Tablet) Information". FDA.
- 1 2 Ortiz-Gómez T, Santesmases MJ (22 April 2016). Gendered Drugs and Medicine: Historical and Socio-Cultural Perspectives. Taylor & Francis. pp. 175–. ISBN 978-1-317-12981-3.
The 1966 marketing campaign for Schering's second contraceptive, Eugynon, [...] (Schering AG Berline 1966, 11). [...] In 1970 [Schering] had already conducted an opinion poll among doctors in the run up to the marketing campaign for the newly introduced Neogynon. [...]
- 1 2 Pohl WG (2004). Die wissenschaftliche Welt von gestern: die Preisträger des Ignaz L. Lieben-Preises 1865-1937 und des Richard Lieben-Preises 1912-1928: ein Kapitel österreichischer Wissenschaftsgeschichte in Kurzbiografien. Böhlau Verlag Wien. pp. 150–. ISBN 978-3-205-77303-0. Archived from the original on 2023-01-12. Retrieved 2018-04-18.
[The contraceptive Eugynon is launched in 1966. Neogynon follows in 1970.]
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 479. ISBN 9783527607495.
- ↑ Carp HJ (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. p. 112. ISBN 978-3-319-14385-9.
- ↑ "Generic Lo/Ovral-28 Availability". Archived from the original on 2019-03-02. Retrieved 2018-03-10.
- ↑ "The Top 300 of 2020". ClinCalc. Archived from the original on 19 March 2022. Retrieved 7 October 2022.
- ↑ "Ethinyl Estradiol; Norgestrel - Drug Usage Statistics". ClinCalc. Archived from the original on 7 October 2021. Retrieved 7 October 2022.
- ↑ Yuzpe AA, Smith RP, Rademaker AW (April 1982). "A multicenter clinical investigation employing ethinyl estradiol combined with dl-norgestrel as postcoital contraceptive agent". Fertility and Sterility. 37 (4): 508–513. doi:10.1016/s0015-0282(16)46157-1. PMID 7040117.
- 1 2 Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ↑ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- 1 2 Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9. Archived from the original on 11 January 2023. Retrieved 13 August 2022.
- ↑ Leidenberger FA, Strowitzki T, Ortmann O (29 August 2009). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 225, 227. ISBN 978-3-540-89760-6. Archived from the original on 14 July 2023. Retrieved 13 August 2022.
- 1 2 Die Gestagene. Springer-Verlag. 27 November 2013. pp. 16–17, 284–. ISBN 978-3-642-99941-3. Archived from the original on 14 July 2023. Retrieved 19 September 2018.
- ↑ Alldredge BK, Corelli RL, Ernst ME (1 February 2012). Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins. pp. 1072–. ISBN 978-1-60913-713-7. Archived from the original on 12 January 2023. Retrieved 3 August 2017.
- ↑ Lavery JP, Sanfilippo JS (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 248–. ISBN 978-1-4612-5064-7. Archived from the original on 12 January 2023. Retrieved 3 August 2017.
- ↑ Offermanns S, Rosenthal W (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 390–. ISBN 978-3-540-38916-3.
- 1 2 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- ↑ Marks L (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7.