 | |
| Names | |
|---|---|
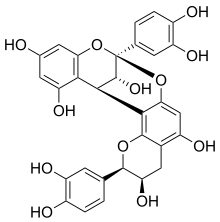
| IUPAC name
(2R,3R,8S,14R,15R)-2,8-bis(3,4-dihydroxyphenyl)-2,3,4,14-tetrahydro-8,14-methanobenzo[7,8][1,3]dioxocino[4,5-h]chromene-3,5,11,13,15-pentaol | |
| Other names
Dimeric catechin Procyanidin A2 Procyanidol A2 Proanthocyanidin A-2 Procyanidin dimer A2 (+)-Proanthocyanidin A2 Epicatechin-(2β→7,4β→8)-epicatechin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H24O12 | |
| Molar mass | 576.510 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Procyanidin A2 is an A type proanthocyanidin.
It is found in avocado,[1] chestnut,[2][3] cranberry juice concentrate,[4] lychee fruit pericarp,[5] peanut[4] skins,[6] Cinchona cortex, cinnamon cortex, Urvillea ulmaceae,[7] and Ecdysanthera utilis.[8]
Synthesis
Procyanidin B2 can be converted into procyanidin A2 by radical oxidation using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals under neutral conditions.[9]
References
- ↑ Proanthocyanidin-A-2 on liberherbarum.com
- ↑ Facino, R. Maffei; Carini, M.; Brambilla, A.; Bombardelli, E.; Morazzoni, P. (1996). "Proanthocyanidin-A2: a new polyphenol". Cosmetics & Toiletries.
- ↑ Kimura, H; Ogawa, S; Akihiro, T; Yokota, K (2011). "Structural analysis of A-type or B-type highly polymeric proanthocyanidins by thiolytic degradation and the implication in their inhibitory effects on pancreatic lipase". J Chromatogr A. 1218 (42): 7704–12. doi:10.1016/j.chroma.2011.07.024. PMID 21803362.
- 1 2 Koerner Jayma, Hsu Victor, Lee Jungmin, Kennedy, James (2009). "Determination of Proanthocyanidin A2 Content in Phenolic Polymer Isolates by Reversed-Phase High Performance Liquid Chromatography". Journal of Chromatography A. 1216 (9): 1403–1409. doi:10.1016/j.chroma.2008.12.086. PMID 19168185.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Sarni-Manchado P, Le Roux E, Le Guerneve C, Lozano Y, Cheynier V. Phenolic composition of litchi fruit pericarp" J Agric Food Chem 2000;48(12):5995-6002.
- ↑ Hongxiang Lou; Yamazaku Y.; Sasaku T.; Uchida M.; Tanaka H.; Oka S. (1999). "A-type proanthocyanidins from peanut skins". Phytochemistry. 51 (2): 297–308. doi:10.1016/S0031-9422(98)00736-5.
- ↑ Dias, Suziane A.; Cardoso (Gazio), Flávia P.; Santin, Silvana M. O.; Da Costa, Willian F.; Vidotti, Gentil J.; De Souza, Maria Conceição; Sarragiotto, Maria Helena (2009). "Free radical scavenging activity and chemical constituents of Urvillea ulmaceae". Pharmaceutical Biology. 47 (8): 717–720. doi:10.1080/13880200902933336. S2CID 54906659.
- ↑ Lin, Lie-Chwen; Kuo, Yuh-Chi; Chou, Cheng-Jen (2002). "Immunomodulatory Proanthocyanidins from Ecdysantherautilis". Journal of Natural Products. 65 (4): 505–8. doi:10.1021/np010414l. PMID 11975489.
- ↑ Kondo, Kazunari; Kurihara, Masaaki; Fukuhara, Kiyoshi; Tanaka, Takashi; Suzuki, Takashi; Miyata, Naoki; Toyoda, Masatake (2000). "Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation". Tetrahedron Letters. 41 (4): 485–488. doi:10.1016/S0040-4039(99)02097-3.
- Wen, LR; Wu, D; Jiang, YM; Prasad, KN; Lin, S; Jiang, GX; He, JR; Zhao, MM; Luo, W; Yang, B (2014). "Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities". Journal of Functional Foods. 6: 555–563. doi:10.1016/j.jff.2013.11.022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.