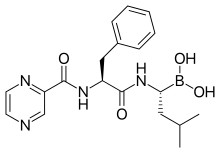
Chemical structure of bortezomib, the first proteasome inhibitor approved for use.
Proteasome inhibitors (INN stem –zomib)[1] are drugs that block the action of proteasomes, cellular complexes that break down proteins. They are being studied in the treatment of cancer; three are approved for use in treating multiple myeloma.
Mechanism
Multiple mechanisms are likely to be involved, but proteasome inhibition may prevent degradation of pro-apoptotic factors such as the p53 protein, permitting activation of programmed cell death in neoplastic cells dependent upon suppression of pro-apoptotic pathways. For example, bortezomib causes a rapid and dramatic change in the levels of intracellular peptides.[2]
Examples
- The first non-peptidic proteasome inhibitor discovered was the natural product lactacystin.[3]
- Disulfiram has been proposed as another proteasome inhibitor.[4][5][6]
- Epigallocatechin-3-gallate has also been proposed.[7]
- Marizomib (salinosporamide A) has started clinical trials for multiple myeloma.
- Oprozomib (ONX-0912), delanzomib (CEP-18770) have also started clinical trials.[8]
- Epoxomicin is a naturally occurring selective inhibitor.[9]
- MG132 is a synthesized peptide commonly used for in vitro studies.
- Beta-hydroxy beta-methylbutyrate is a proteasome inhibitor in human skeletal muscle[10][11] in vivo.[12]
Approved medications
- Bortezomib (Velcade) was approved in 2003. This was the first proteasome inhibitor approved for use in the U.S. Its boron atom binds the catalytic site of the 26S proteasome.[13]
- Carfilzomib (Kyprolis) was approved by the FDA for relapsed and refractory multiple myeloma in 2012 .[14] It irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S proteasome.
- Ixazomib (Ninlaro) was approved by the FDA in 2015 for use in combination with lenalidomide and dexamethasone for the treatment of multiple myeloma after at least one prior therapy. It is the first orally-available proteasome inhibitor [15]
References
- ↑ "The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances" (PDF). World Health Organization. Retrieved 5 November 2016.
- ↑ Gelman JS, Sironi J, Berezniuk I, Dasgupta S, Castro LM, Gozzo FC, Ferro ES, Fricker LD (2013). "Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib". PLOS ONE. 8 (1): e53263. Bibcode:2013PLoSO...853263G. doi:10.1371/journal.pone.0053263. PMC 3538785. PMID 23308178.
- ↑ Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL (1995). "Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin". Science. 268 (5211): 726–31. Bibcode:1995Sci...268..726F. doi:10.1126/science.7732382. PMID 7732382. S2CID 37779687.
- ↑ Lövborg H, Oberg F, Rickardson L, Gullbo J, Nygren P, Larsson R (March 2006). "Inhibition of proteasome activity, nuclear factor-KappaB translocation and cell survival by the antialcoholism drug disulfiram". International Journal of Cancer. 118 (6): 1577–80. doi:10.1002/ijc.21534. PMID 16206267.
- ↑ Wickström M, Danielsson K, Rickardson L, et al. (January 2007). "Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients". Biochemical Pharmacology. 73 (1): 25–33. doi:10.1016/j.bcp.2006.08.016. PMID 17026967.
- ↑ Cvek B, Dvorak Z (August 2008). "The value of proteasome inhibition in cancer. Can the old drug, disulfiram, have a bright new future as a novel proteasome inhibitor?". Drug Discovery Today. 13 (15–16): 716–22. doi:10.1016/j.drudis.2008.05.003. PMID 18579431.
- ↑ Osanai K, Landis-Piwowar KR, Dou QP, Chan TH (August 2007). "A para-amino substituent on the D-ring of green tea polyphenol epigallocatechin-3-gallate as a novel proteasome inhibitor and cancer cell apoptosis inducer". Bioorg. Med. Chem. 15 (15): 5076–82. doi:10.1016/j.bmc.2007.05.041. PMC 2963865. PMID 17544279.
- ↑ "Current Advances in Novel Proteasome Inhibitor–Based Approaches to the Treatment of Relapsed/Refractory Multiple Myeloma". 2011.
- ↑ Meng, L.; et al. (1999). "Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity". Proc. Natl. Acad. Sci. U.S.A. 96 (18): 10403–10408. Bibcode:1999PNAS...9610403M. doi:10.1073/pnas.96.18.10403. PMC 17900. PMID 10468620.
- ↑ Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". J. Int. Soc. Sports Nutr. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
Skeletal muscle proteolysis is increased in catabolic states such as fasting, immobilization, aging, and disease [77]. HMB has been shown to decrease skeletal muscle protein degradation both in vitro[72,73] and in vivo[78]. ... Indeed, HMB has been shown to decrease proteasome expression [72] and activity [72,78-80] during catabolic states, thus attenuating skeletal muscle protein degradation through the ubiquitin-proteasome pathway.
- ↑ Luckose F, Pandey MC, Radhakrishna K (2015). "Effects of amino acid derivatives on physical, mental, and physiological activities". Crit. Rev. Food Sci. Nutr. 55 (13): 1793–1807. doi:10.1080/10408398.2012.708368. PMID 24279396. S2CID 22657268.
HMB, a derivative of leucine, prevents muscle damage and increases muscle strength by reducing exercise-induced proteolysis in muscles and also helps in increasing lean body mass.
- ↑ Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ (June 2013). "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism". J. Physiol. 591 (11): 2911–2923. doi:10.1113/jphysiol.2013.253203. PMC 3690694. PMID 23551944.
although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ~50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008).
- ↑ Bonvini P, Zorzi E, Basso G, Rosolen A (2007). "Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma". Leukemia. 21 (4): 838–42. doi:10.1038/sj.leu.2404528. PMID 17268529.
- ↑ "Press Announcements — FDA approves Kyprolis for some patients with multiple myeloma". U.S. Food and Drug Administration. July 20, 2012. Retrieved 24 April 2016.
- ↑ "Press Announcements — FDA approves Ninlaro, new oral medication to treat multiple myeloma". U.S. Food and Drug Administration. Retrieved 24 April 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.