 | |
| Names | |
|---|---|
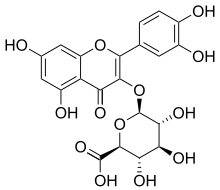
| IUPAC name
3′,4′,5,7-Tetrahydroxy-4-oxoflav-2-en-3-yl β-D-glucopyranosiduronic acid | |
| Systematic IUPAC name
(2S,3S,4S,5R,6S)-6-{[2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-1-benzopyran-3-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| Other names
Quercetin 3-O-glucuronide Quercetol glucuronide Quercetin 3-glucuronide Quercetol 3-O-glucuronide quercetin 3-O-β-D-glucuronopyranoside Querciturone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C21H18O13 | |
| Molar mass | 478.362 g·mol−1 |
| Density | 1.961 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Miquelianin (quercetin 3-O-glucuronide) is a flavonol glucuronide, a type of phenolic compound present in wine,[1] in species of St John's wort,[2] like Hypericum hirsutum,[3] in Nelumbo nucifera (Indian lotus)[4] or in green beans.[5]
It is also a rat plasma quercetin metabolite.[6] It shows an antioxidant effect in human plasma.[7] In vitro studies indicate that miquelianin is able to reach the central nervous system from the small intestine.[8]
See also
References
- ↑ Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. (1998). "Antioxidant Activity of Different Phenolic Fractions Separated from an Italian Red Wine". Journal of Agricultural and Food Chemistry. 46 (2): 361–367. doi:10.1021/jf970486b. PMID 10554247.
- ↑ Wei, Y.; Xie, Q.; Dong, W.; Ito, Y. (2009). "Separation of epigallocatechin and flavonoids from Hypericum perforatum L. By high-speed counter-current chromatography and preparative high-performance liquid chromatography". Journal of Chromatography A. 1216 (19): 4313–4318. doi:10.1016/j.chroma.2008.12.056. PMC 2777726. PMID 19150073.
- ↑ Kitanov, G. M. (1988). "Miquelianin and other polyphenols from Hypericum hirsutum". Chemistry of Natural Compounds. 24: 119–120. doi:10.1007/BF00597593. S2CID 37846890.
- ↑ Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y. P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L. M.; Morris-Natschke, S. L.; Lee, K. H. (2005). "Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure–activity correlations with related alkaloids". Bioorganic & Medicinal Chemistry. 13 (2): 443–448. doi:10.1016/j.bmc.2004.10.020. PMID 15598565.
- ↑ Plumb, G. W.; Price, K. R.; Williamson, G. (1999). "Antioxidant properties of flavonol glycosides from green beans". Redox Report. 4 (3): 123–127. doi:10.1179/135100099101534800. PMID 10496415.
- ↑ Moon, J. H.; Tsushida, T.; Nakahara, K.; Terao, J. (2001). "Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin". Free Radical Biology and Medicine. 30 (11): 1274–1285. doi:10.1016/S0891-5849(01)00522-6. PMID 11368925.
- ↑ Terao, J.; Yamaguchi, S.; Shirai, M.; Miyoshi, M.; Moon, J. H.; Oshima, S.; Inakuma, T.; Tsushida, T.; Kato, Y. (2001). "Protection by quercetin and quercetin 3-O-β-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein". Free Radical Research. 35 (6): 925–931. doi:10.1080/10715760100301421. PMID 11811543. S2CID 22095635.
- ↑ Juergenliemk, G.; Boje, K.; Huewel, S.; Lohmann, C.; Galla, H. J.; Nahrstedt, A. (2003). "In VitroStudies Indicate that Miquelianin (Quercetin 3-O-ß-D-Glucuronopyranoside) is Able to Reach the CNS from the Small Intestine". Planta Medica. 69 (11): 1013–1017. doi:10.1055/s-2003-45148. PMID 14735439.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.