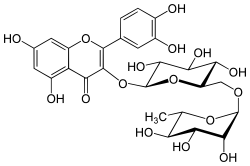
 | |
| Names | |
|---|---|
| IUPAC name
3′,4′,5,7-Tetrahydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavone | |
| Systematic IUPAC name
(42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-13,14,25,27,43,44,45,73,74,75-Decahydroxy-76-methyl-24H-3,6-dioxa-2(2,3)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphane-24-one | |
| Other names
Rutoside (INN) Phytomelin Sophorin Birutan Eldrin Birutan Forte Rutin trihydrate Globularicitrin Violaquercitrin Quercetin rutinoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.287 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H30O16 | |
| Molar mass | 610.521 g·mol−1 |
| Appearance | Solid |
| Melting point | 242 °C (468 °F; 515 K) |
| 12.5 mg/100 mL[1] 13 mg/100mL[2] | |
| Pharmacology | |
| C05CA01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Rutin (rutoside, quercetin-3-O-rutinoside or sophorin) is the glycoside combining the flavonol quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose). It is a flavonoid glycoside found in a wide variety of plants, including citrus.
Occurrences
Rutin is one of the phenolic compounds found in the invasive plant species, Carpobrotus edulis. Its name comes from the name of Ruta graveolens, a plant that also contains rutin. Various citrus fruit peels contain 32 to 49 mg/g of flavonoids expressed as rutin equivalents.[3] Citrus leaves contain rutin at concentrations of 11 and 7 g/kg in orange and lime trees, respectively.[4] In 2021, Samoan researchers identified rutin in the native plant matalafi (Psychotria insularum).[5]
Metabolism
The enzyme quercitrinase found in Aspergillus flavus is in the rutin catabolic pathway.[6]
In food
Rutin is a citrus flavonoid glycoside found in many plants, including buckwheat,[7] the leaves and petioles of Rheum species, and asparagus. Tartary buckwheat seeds have been found to contain more rutin (about 0.8–1.7% dry weight) than common buckwheat seeds (0.01% dry weight).[7] Rutin is one of the primary flavonols found in 'clingstone' peaches.[8] It is also found in green tea infusions.[9]
Approximate rutin content per 100g of selected foods, in milligrams per 100 milliliters:[10]
| Numeric | Alphabetic |
|---|---|
| 332 | Capers, spice |
| 45 | Olive (black), raw |
| 36 | Buckwheat, whole grain flour |
| 23 | Asparagus, raw |
| 19 | Black raspberry, raw |
| 11 | Red raspberry, raw |
| 9 | Buckwheat, groats, thermally treated |
| 6 | Buckwheat, refined flour |
| 6 | Greencurrant |
| 6 | Plum, fresh |
| 5 | Blackcurrant, raw |
| 4 | Blackberry, raw |
| 3 | Tomato (cherry), whole, raw |
| 2 | Prune |
| 2 | Fenugreek |
| 2 | Marjoram, dried |
| 2 | Tea (black), infusion |
| 1 | Grape, raisin |
| 1 | Zucchini, raw |
| 1 | Apricot, raw |
| 1 | Tea (green), infusion |
| 0 | Apple |
| 0 | Redcurrant |
| 0 | Grape (green) |
| 0 | Tomato, whole, raw |
Research
Rutin (rutoside or rutinoside)[11] and other dietary flavonols are under preliminary clinical research for their potential biological effects, such as in reducing post-thrombotic syndrome, venous insufficiency, or endothelial dysfunction, but there was no high-quality evidence for their safe and effective uses as of 2018.[11][12][13] A 2020 review indicated that oral rutosides may reduce leg edema in people with post-thrombotic syndrome, but the risk of adverse effects was higher.[14]
As a flavonol among similar flavonoids, rutin has low bioavailability due to poor absorption, high metabolism, and rapid excretion that collectively make its potential for use as a therapeutic agent limited.[11]
Biosynthesis
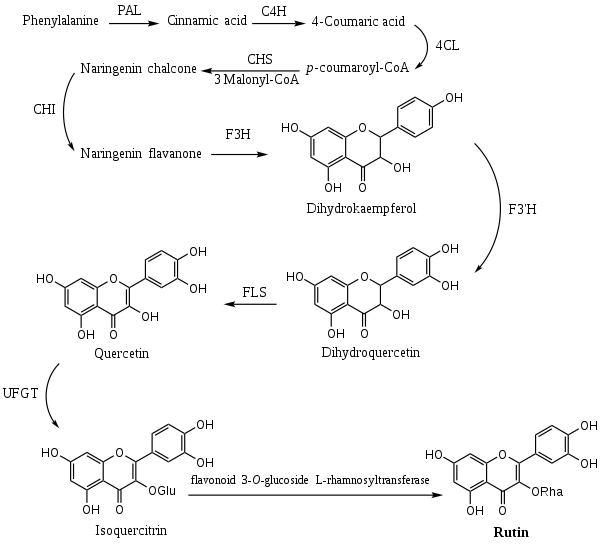
The biosynthesis pathway of rutin in mulberry (Morus alba L.) leaves begins with phenylalanine, which produces cinnamic acid under the action of phenylalanine ammonia lyase (PAL). Cinnamic acid is catalyzed by cinnamic acid-4-hydroxylase (C4H) and 4-coumarate-CoA ligase (4CL) to form p-coumaroyl-CoA. Subsequently, chalcone synthase (CHS) catalyzes the condensation of p-coumaroyl-CoA and three molecules of malonyl-CoA to produce naringenin chalcone, which is eventually converted into naringenin flavanone with the participation of chalcone isomerase (CHI). With the action of flavanone 3-hydroxylas (F3H), dihydrokaempferol (DHK) is generated. DHK can be further hydroxylated by flavonoid 3´-hydroxylase (F3'H) to produce dihydroquercetin (DHQ), which is then catalyzed by flavonol synthase (FLS) to form quercetin. After quercetin is catalyzed by UDP-glucose flavonoid 3-O-glucosyltransferase (UFGT) to form isoquercitrin, finally, the formation of rutin from isoquercitrin is catalyzed by flavonoid 3-O-glucoside L-rhamnosyltransferase.[15]
References
- ↑ Merck Index, 12th Edition, 8456
- ↑ Krewson CF, Naghski J (November 1952). "Some physical properties of rutin". Journal of the American Pharmaceutical Association. 41 (11): 582–587. doi:10.1002/jps.3030411106. PMID 12999623.
- ↑ Wang, Yuan-Chuen; Chuang, Yueh-Chueh; Hsu, Hsing-Wen (2008). "The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan". Food Chemistry. 106 (1): 277–284. doi:10.1016/j.foodchem.2007.05.086. ISSN 0308-8146.
- ↑ Soares, Márcio Santos; da Silva, Danielle Fernandes; Forim, Moacir Rossi; da Silva, Maria Fátima das Graças Fernandes; Fernandes, João Batista; Vieira, Paulo Cezar; Silva, Denise Brentan; Lopes, Norberto Peporine; de Carvalho, Sérgio Alves; de Souza, Alessandra Alves; Machado, Marcos Antônio (2015). "Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC-UV and MALDI imaging mass spectrometry". Phytochemistry. 115: 161–170. Bibcode:2015PChem.115..161S. doi:10.1016/j.phytochem.2015.02.011. ISSN 0031-9422. PMID 25749617.
- ↑ Molimau-Samasoni S, Woolner VH, Foliga ST, Robichon K, Patel V, Andreassend SK, et al. (November 2021). "Functional genomics and metabolomics advance the ethnobotany of the Samoan traditional medicine "matalafi"". Proceedings of the National Academy of Sciences of the United States of America. 118 (45): e2100880118. Bibcode:2021PNAS..11800880M. doi:10.1073/pnas.2100880118. PMC 8609454. PMID 34725148. S2CID 240423413.
- ↑ Tranchimand S, Brouant P, Iacazio G (November 2010). "The rutin catabolic pathway with special emphasis on quercetinase". Biodegradation. 21 (6): 833–859. doi:10.1007/s10532-010-9359-7. PMID 20419500. S2CID 30101803.
- 1 2 Kreft S, Knapp M, Kreft I (November 1999). "Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis". Journal of Agricultural and Food Chemistry. 47 (11): 4649–4652. doi:10.1021/jf990186p. PMID 10552865.
- ↑ Chang S, Tan C, Frankel EN, Barrett DM (February 2000). "Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars". Journal of Agricultural and Food Chemistry. 48 (2): 147–151. doi:10.1021/jf9904564. PMID 10691607.
- ↑ Malagutti AR, Zuin V, Cavalheiro ÉT, Henrique Mazo L (2006). "Determination of Rutin in Green Tea Infusions Using Square-Wave Voltammetry with a Rigid Carbon-Polyurethane Composite Electrode". Electroanalysis. 18 (10): 1028–1034. doi:10.1002/elan.200603496.
- ↑ "foods in which the polyphenol Quercetin 3-O-rutinoside is found". Phenol-Explorer v 3.6. June 2015.
- 1 2 3 "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, Oregon. November 2015. Retrieved 25 February 2018.
- ↑ Morling JR, Broderick C, Yeoh SE, Kolbach DN (November 2018). "Rutosides for treatment of post-thrombotic syndrome". The Cochrane Database of Systematic Reviews. 2018 (11): CD005625. doi:10.1002/14651858.CD005625.pub4. PMC 6517027. PMID 30406640.
- ↑ Martinez-Zapata, Maria José; Vernooij, Robin Wm; Simancas-Racines, Daniel; et al. (2020-11-03). "Phlebotonics for venous insufficiency". The Cochrane Database of Systematic Reviews. 2020 (11): CD003229. doi:10.1002/14651858.CD003229.pub4. ISSN 1469-493X. PMC 8094625. PMID 33141449.
- ↑ Martinez-Zapata, Maria José; Vernooij, Robin Wm; Simancas-Racines, Daniel; et al. (3 November 2020). "Phlebotonics for venous insufficiency". The Cochrane Database of Systematic Reviews. 2020 (11): CD003229. doi:10.1002/14651858.CD003229.pub4. ISSN 1469-493X. PMC 8094625. PMID 33141449.
- ↑ Yu X, Liu J, Wan J, Zhao L, Liu Y, Wei Y, Ouyang Z. Cloning, prokaryotic expression, and enzyme activity of a UDP-glucose flavonoid 3-o-glycosyltransferase from mulberry (Morus alba L.) leaves. Phcog Mag 2020;16:441-7
External links
 Media related to Rutin at Wikimedia Commons
Media related to Rutin at Wikimedia Commons
