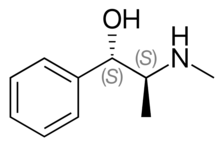 | |
-Pseudoephedrine_molecule_ball.png.webp) | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsuːdoʊ.ɪˈfɛdrɪn, -ˈɛfɪdriːn/ |
| Trade names | Afrinol, Sudafed, Sinutab, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682619 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100%[2] |
| Metabolism | 10–30% liver |
| Elimination half-life | 4.3–8 hours[2] |
| Excretion | 43–96% kidney[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.835 |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.236 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant,[3] as a stimulant, or as a wakefulness-promoting agent[4] in higher doses.[5]
It was first characterized in 1889 by the German chemists Ladenburg and Oelschlägel, who used a sample that had been isolated from Ephedra vulgaris by the Merck pharmaceutical corporation of Darmstadt, Germany.[6] The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in over-the-counter preparations, either as a single ingredient or (more commonly) in a fixed-dose combination with one or more additional active ingredients such as antihistamines, guaifenesin, dextromethorphan, paracetamol (acetaminophen), or an NSAID (such as aspirin or ibuprofen).
Medical uses
Pseudoephedrine is a stimulant, but it is well known for shrinking swollen nasal mucous membranes, so it is often used as a decongestant. It reduces tissue hyperemia, edema, and nasal congestion commonly associated with colds or allergies. Other beneficial effects may include increasing the drainage of sinus secretions, and opening of obstructed Eustachian tubes. The same vasoconstriction action can also result in hypertension, which is a noted side effect of pseudoephedrine.
Pseudoephedrine can be used either as oral or as topical decongestant. Due to its stimulating qualities, however, the oral preparation is more likely to cause adverse effects, including urinary retention.[7][8] According to one study, pseudoephedrine may show effectiveness as an antitussive drug (suppression of cough).[9]
Pseudoephedrine is indicated for the treatment of nasal congestion, sinus congestion, and Eustachian tube congestion.[10] Pseudoephedrine is also indicated for vasomotor rhinitis, and as an adjunct to other agents in the optimum treatment of allergic rhinitis, croup, sinusitis, otitis media, and tracheobronchitis.[10]
Pseudoephedrine is also used as a first-line prophylactic for recurrent priapism. Erection is largely a parasympathetic response, so the sympathetic action of pseudoephedrine may serve to relieve this condition. Treatment for urinary incontinence is an off-label use ("unlabeled use") for these medications.[11][12]
Adverse effects
Common adverse drug reactions (ADRs) associated with pseudoephedrine therapy include central nervous system stimulation, insomnia, nervousness, excitability, dizziness and anxiety. Infrequent ADRs include tachycardia or palpitations. Rarely, pseudoephedrine therapy may be associated with mydriasis (dilated pupils), hallucinations, arrhythmias, hypertension, seizures and ischemic colitis;[13] as well as severe skin reactions known as recurrent pseudo-scarlatina, systemic contact dermatitis, and nonpigmenting fixed drug eruption.[14] Pseudoephedrine, particularly when combined with other drugs including narcotics, may also play a role in the precipitation of episodes of paranoid psychosis.[15] It has also been reported that pseudoephedrine, among other sympathomimetic agents, may be associated with the occurrence of stroke.[16]
Precautions and contraindications
Pseudoephedrine is contraindicated in patients with diabetes mellitus, cardiovascular disease, severe or uncontrolled hypertension, severe coronary artery disease, prostatic hypertrophy, hyperthyroidism, closed angle glaucoma, or by pregnant women.[13] The safety and effectiveness of nasal decongestant use in children is unclear.[17]
Interactions
Concomitant or recent (previous fourteen days) monoamine oxidase inhibitor use can lead to hypertensive reactions, including hypertensive crises.[13]
The antihypertensive effects of methyldopa, mecamylamine, reserpine, and veratrum alkaloids may be reduced by sympathomimetics. Beta-adrenergic antagonists may also interact with sympathomimetics. Increase of ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Antacids increase the rate of pseudoephedrine absorption, while kaolin decreases it.
Mechanism of action
Pseudoephedrine is a sympathomimetic amine. Its principal mechanism of action relies on its direct action on the adrenergic receptor system.[18][19] The vasoconstriction that pseudoephedrine produces is believed to be principally an α-adrenergic receptor response.[20]
Pseudoephedrine acts on α- and β2-adrenergic receptors, to cause vasoconstriction and relaxation of smooth muscle in the bronchi, respectively.[18][19] α-Adrenergic receptors are located on the muscles lining the walls of blood vessels. When these receptors are activated, the muscles contract, causing the blood vessels to constrict (vasoconstriction). The constricted blood vessels now allow less fluid to leave the blood vessels and enter the nose, throat, and sinus linings, which results in decreased inflammation of nasal membranes, as well as decreased mucus production. Thus, by constriction of blood vessels, mainly those located in the nasal passages, pseudoephedrine causes a decrease in the symptoms of nasal congestion. Activation of β2-adrenergic receptors produces relaxation of smooth muscle of the bronchi,[18] causing bronchial dilation and in turn decreasing congestion (although not fluid) and difficulty breathing.
Other uses
There have been reports of off-label uses of pseudoephedrine for its stimulant properties. Long-distance truck drivers and athletes, for example, have reportedly used pseudoephedrine as a stimulant to increase their state of alertness/awareness.
A study has also found that pseudoephedrine can reduce milk production in breastfeeding women.[21]
Manufacture of amphetamines
Its membership in the amphetamine class has made pseudoephedrine a sought-after chemical precursor in the illicit manufacture of methamphetamine and methcathinone. As a result of the increasing regulatory restrictions on the sale and distribution of pseudoephedrine, pharmaceutical firms have reformulated medications to use alternative compounds, particularly phenylephrine, even though its efficacy as an oral decongestant has been demonstrated to be indistinguishable from placebo.[22]
In the United States, federal laws control the sale of pseudoephedrine-containing products.[23][24][25] Retailers in the US have created corporate policies restricting the sale of pseudoephedrine-containing products.[26][27] Their policies restrict sales by limiting purchase quantities and requiring a minimum age and government issued photographic identification.[24][25] These requirements are similar to and sometimes more stringent than existing law. Internationally, pseudoephedrine is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[28]
Sports
Pseudoephedrine was on the International Olympic Committee's (IOC) banned substances list until 2004, when the World Anti-Doping Agency (WADA) list replaced the IOC list. Although WADA initially only monitored pseudoephedrine, it went back onto the "banned" list on 1 January 2010.[29]
Pseudoephedrine is excreted through urine, and concentration in urine of this drug shows a large inter-individual spread; that is, the same dose can give a vast difference in urine concentration for different individuals.[30] Pseudoephedrine is approved to be taken up to 240 mg per day. In seven healthy male subjects this dose yielded a urine concentration range of 62.8 to 294.4 microgram per milliliter (µg/mL) with mean ± standard deviation 149 ± 72 µg/mL.[31] Thus, normal dosage of 240 mg pseudoephedrine per day can result in urine concentration levels exceeding the limit of 150 µg/mL set by WADA for about half of all users.[32] Furthermore, hydration status does not affect urinary concentration of pseudoephedrine.[33]
Canadian rower Silken Laumann was stripped of her 1995 Pan American Games team gold medal after testing positive for pseudoephedrine.[34]
In February 2000, Elena Berezhnaya and Anton Sikharulidze won gold at the 2000 European Figure Skating Championships but were stripped of their medals after Berezhnaya tested positive. This resulted in a three-month disqualification from the date of the test, and the medal being stripped.[35] She stated that she had taken cold medication approved by a doctor but had failed to inform the ISU as required.[36] The pair missed the World Championships that year as a result of the disqualification.
Romanian gymnast Andreea Răducan was stripped of her gold medal at the 2000 Summer Olympic Games after testing positive. She took two pills given to her by the team coach for a cold. Although she was stripped of the overall gold medal, she kept her other medals, and, unlike in most other doping cases, was not banned from competing again; only the team doctor was banned for a number of years. Ion Țiriac, the president of the Romanian Olympic Committee, resigned over the scandal.[37][38]
In the 2010 Winter Olympic Games, the IOC issued a reprimand against the Slovak ice hockey player Lubomir Visnovsky for usage of pseudoephedrine.[39]
In the 2014 Winter Olympic Games Team Sweden and Washington Capitals ice hockey player Nicklas Bäckström was prevented from playing in the final for usage of pseudoephedrine. Bäckström claimed he was using it as allergy medication.[40] In March 2014, the IOC Disciplinary Commission decided that Bäckström would be awarded the silver medal.[41] In January 2015 Bäckström, the IOC, WADA and the IIHF agreed to a settlement in which he accepted a reprimand but was cleared of attempting to enhance his performance.[42]
Detection of use
Pseudoephedrine may be quantified in blood, plasma, or urine to monitor any possible performance-enhancing use by athletes, confirm a diagnosis of poisoning, or to assist in a medicolegal death investigation. Some commercial immunoassay screening tests directed at the amphetamines cross-react appreciably with pseudoephedrine, but chromatographic techniques can easily distinguish pseudoephedrine from other phenethylamine derivatives. Blood or plasma pseudoephedrine concentrations are typically in the 50–300 µg/L range in persons taking the drug therapeutically, 500–3000 µg/L in people with substance use disorder involving pseudoephedrine, or poisoned patients and 10–70 mg/L in cases of acute fatal overdose.[43][44]
Chemistry
Pseudoephedrine is a diastereomer of ephedrine and is readily reduced into methamphetamine or oxidized into methcathinone.
ephedrine_enantiomers.svg.png.webp)
Nomenclatures
The dextrorotary (+)- or d- enantiomer is (1S,2S)-pseudoephedrine, whereas the levorotating (−)- or l- form is (1R,2R)-pseudoephedrine.
In the outdated D/L system (+)-pseudoephedrine is also referred to as L-pseudoephedrine and (−)-pseudoephedrine as D-pseudoephedrine (in the Fisher projection then the phenyl ring is drawn at bottom).[45][46]
Often the D/L system (with small caps) and the d/l system (with lower-case) are confused. The result is that the dextrorotary d-pseudoephedrine is wrongly named D-pseudoephedrine and the levorotary l-ephedrine (the diastereomer) wrongly L-ephedrine.
The IUPAC names of the two enantiomers are (1S,2S)- respectively (1R,2R)-2-methylamino-1-phenylpropan-1-ol. Synonyms for both are psi-ephedrine and threo-ephedrine.
Pseudoephedrine is the International Nonproprietary Name of the (+)-form, when used as pharmaceutical substance.[47]
Society and culture
Brand names
The following is a list of consumer medicines that either contain pseudoephedrine or have switched to a less-regulated alternative such as phenylephrine.
- Actifed (made by GlaxoSmithKline) — contains 60 mg pseudoephedrine and 2.5 mg triprolidine in certain countries.
- Advil Cold & Sinus (made by Pfizer Canada Inc.) — contains 30 mg pseudoephedrine hydrochloride and 200 mg ibuprofen.
- Aleve-D Sinus & Cold (made by Bayer Healthcare) — contains 120 mg pseudoephedrine hydrochloride (also 220 mg naproxen).
- Allegra-D (made by Sanofi Aventis) — contains 120 mg of pseudoephedrine hydrochloride (also 60 mg of fexofenadine).
- Allerclear-D (made by Kirkland Signature) — contains 240 mg of pseudoephedrine sulfate (also 10 mg of loratadine).
- Benadryl Allergy Relief Plus Decongestant (made by McNeil Consumer Healthcare, a Johnson & Johnson company) — contains 60 mg pseudoephedrine hydrochloride (also 8 mg acrivastine)[48]
- Cirrus (made by UCB) — contains 120 mg pseudoephedrine hydrochloride (also 5 mg cetirizine).
- Claritin-D (made by Bayer Healthcare) — contains 120 mg of pseudoephedrine sulfate (also 5 mg of loratadine).
- Claritin-D 24 Hour (made by Bayer Healthcare) — contains 240 mg of pseudoephedrine sulfate (also 10 mg of loratadine).
- Codral (made by Asia-Pacific subsidiary of Johnson & Johnson) — Codral Original contains pseudoephedrine, Codral New Formula substitutes phenylephrine for pseudoephedrine.
- Congestal (made by SIGMA Pharmaceutical Industries) — contains 60 mg pseudoephedrine hydrochloride (also 650 mg paracetamol and 4 mg chlorpheniramine).[49][50]
- Contac (made by GlaxoSmithKline) — previously contained pseudoephedrine, now contains phenylephrine. As at Nov 2014 UK version still contains 30 mg pseudoephedrine hydrochloride per tablet.
- Demazin (made by Bayer Healthcare) — contains pseudoephedrine sulfate and chlorpheniramine maleate
- Eltor (made by Sanofi Aventis) — contains pseudoephedrine hydrochloride.
- Mucinex D (made by Reckitt Benckiser) — contains 60 mg pseudoephedrine hydrochloride (also 1200 mg guaifenesin).
- Nexafed (made by Acura Pharmaceuticals) — contains 30 mg pseudoephedrine per tablet, formulated with Impede Meth-Deterrent technology.
- Nurofen Cold & Flu (made by Reckitt Benckiser) — contains 30 mg pseudoephedrine hydrochloride (also 200 mg ibuprofen).
- Respidina – contains 120 mg of pseudoephedrine in the form of extended release tablets.
- Rhinex Flash (made by Pharma Product Manufacturing, Cambodia) — contains pseudoephedrine combined with paracetamol and triprolidine.
- Rhinos SR (made by Dexa Medica) — contains 120 mg of pseudoephedrine hydrochloride
- Trima — contains 60 mg pseudoephedrine hydrochloride.
- Sinutab (made by McNeil Consumer Healthcare, a Johnson & Johnson company) — contains 500 mg paracetamol and 30 mg pseudoephedrine hydrochloride.
- Sudafed Decongestant (made by McNeil Consumer Healthcare, a Johnson & Johnson company) — contains 60 mg of pseudoephedrine hydrochloride. Not to be confused with Sudafed PE, which contains phenylephrine.
- Theraflu (made by Novartis) — previously contained pseudoephedrine, now contains phenylephrine.
- Unifed (made by United Pharmaceutical Manufacturer, Jordan) — contains pseudoephedrine hydrochloride (also triprolidine and guaifenesin).
- Zyrtec-D 12 Hour (made by McNeil Consumer Healthcare, a Johnson & Johnson company) — contains 120 mg pseudoephedrine hydrochloride (also 5 mg of cetirizine).
- Zephrex-D (made by Westport Pharmaceuticals) – a special meth-resistant form of pseudoephedrine that becomes gooey when heated.
Legal status
Australia

Illicit diversion of pseudoephedrine in Australia has caused significant changes to the way the products are regulated. As of 2006, all products containing pseudoephedrine have been rescheduled as either "Pharmacist Only Medicines" (Schedule 3) or "Prescription Only Medicines" (Schedule 4), depending on the amount of pseudoephedrine in the product. A Pharmacist Only Medicine may only be sold to the public if a pharmacist is directly involved in the transaction. These medicines must be kept behind the counter, away from public access.
Pharmacists are also encouraged (and in some states required) to log purchases with the online database Project STOP.[51]
As a result, some pharmacies no longer stock Sudafed, the common brand of pseudoephedrine cold/sinus tablets, opting instead to sell Sudafed PE, a phenylephrine product that has not been proven effective in clinical trials.[22][52][53]
Belgium
Several formulations of pseudoephedrine are available over-the-counter in Belgium.[54]
Canada
Health Canada has investigated the risks and benefits of pseudoephedrine and ephedrine/Ephedra. Near the end of the study, Health Canada issued a warning on their website stating that those who are under the age of 12, or who have heart disease and may have strokes, should avoid taking pseudoephedrine and ephedrine. Also, they warned that everyone should avoid taking ephedrine or pseudoephrine with other stimulants like caffeine. They also banned all products that contain both ephedrine (or pseudoephedrine) and caffeine.[55]
Products whose only medicinal ingredient is pseudoephedrine must be kept behind the pharmacy counter. Products containing pseudoephedrine along with other medicinal ingredients may be displayed on store shelves but may be sold only in a pharmacy when a pharmacist is present.[56][57]
Colombia
The Colombian government prohibited the trade of pseudoephedrine in 2010.[58]
Estonia
Pseudoephedrine is an over-the-counter drug in Estonia.[59]
Finland
Pseudoephedrine medicines can only be obtained with a prescription in Finland.[60]
Japan
Medications that contain more than 10% pseudoephedrine are prohibited under the Stimulants Control Law in Japan.[61]
Mexico
On 23 November 2007, the use and trade of pseudoephedrine in Mexico was made illegal as it was argued that it was extremely popular as a precursor in the synthesis of methamphetamine.[62]
Netherlands
Pseudoephedrine was withdrawn from sale in 1989 due to concerns about adverse cardiac side effects.[63]
New Zealand
In New Zealand, pseudoephedrine is currently classified as a Class B Part II controlled drug in the Misuse of Drugs Act 1975, making it illegal to supply or possess except on prescription.[64][65]
Pseudoephedrine, ephedrine, and any product containing these substances, e.g. cold and flu medicines, were first classified in October 2004 as Class C Part III (partially exempted) controlled drugs, due to being the principal ingredient in methamphetamine.[66] New Zealand Customs and police officers continued to make large interceptions of precursor substances believed to be destined for methamphetamine production. On 9 October 2009, Prime Minister John Key announced pseudoephedrine-based cold and flu tablets would become prescription-only drugs and reclassified as a class B2 drug.[67] The law was amended by The Misuse of Drugs Amendment Bill 2010, which passed in August 2011.[68]
Turkey
In Turkey, medications containing pseudoephedrine are available with prescription only.[69]
United Kingdom
In the UK, pseudoephedrine is available over the counter under the supervision of a qualified pharmacist, or on prescription. In 2007, the MHRA reacted to concerns over diversion of ephedrine and pseudoephedrine for the illicit manufacture of methamphetamine by introducing voluntary restrictions limiting over the counter sales to one box containing no more than 720 mg of pseudoephedrine in total per transaction. These restrictions became law in April 2008.[70] No form of ID is required.
United States
Federal
The United States Congress has recognized that pseudoephedrine is used in the illegal manufacture of methamphetamine. In 2005, the Committee on Education and the Workforce heard testimony concerning education programs and state legislation designed to curb this illegal practice.
Attempts to control the sale of the drug date back to 1986, when federal officials at the Drug Enforcement Administration (DEA) first drafted legislation, later proposed by Senator Bob Dole, that would have placed a number of chemicals used in the manufacture of illicit drugs under the Controlled Substances Act. The bill would have required each transaction involving pseudoephedrine to be reported to the government, and federal approval of all imports and exports. Fearing this would limit legitimate use of the drug, lobbyists from over the counter drug manufacturing associations sought to stop this legislation from moving forward, and were successful in exempting from the regulations all chemicals that had been turned into a legal final product, such as Sudafed.[71]
Prior to the passage of the Combat Methamphetamine Epidemic Act of 2005, sales of the drug became increasingly regulated, as DEA regulators and pharmaceutical companies continued to fight for their respective positions. The DEA continued to make greater progress in their attempts to control pseudoephedrine as methamphetamine production skyrocketed, becoming a serious problem in the western United States. When purity dropped, so did the number of people in rehab and people admitted to emergency rooms with methamphetamine in their systems. This reduction in purity was usually short lived, however, as methamphetamine producers eventually found a way around the new regulations.[72]
Congress passed the Combat Methamphetamine Epidemic Act of 2005 (CMEA) as an amendment to the renewal of the USA Patriot Act.[24] Signed into law by president George W. Bush on 6 March 2006,[23] the act amended 21 U.S.C. § 830, concerning the sale of pseudoephedrine-containing products. The law mandated two phases, the first needing to be implemented by 8 April 2006, and the second to be completed by 30 September 2006. The first phase dealt primarily with implementing the new buying restrictions based on amount, while the second phase encompassed the requirements of storage, employee training, and record keeping.[73] Though the law was mainly directed at pseudoephedrine products it also applies to all over-the-counter products containing ephedrine, pseudoephedrine, and phenylpropanolamine, their salts, optical isomers, and salts of optical isomers.[73] Pseudoephedrine was defined as a "scheduled listed chemical product" under 21 U.S.C. § 802(45(A)). The act included the following requirements for merchants ("regulated sellers") who sell such products:
- Required a retrievable record of all purchases, identifying the name and address of each party, to be kept for two years
- Required verification of proof of identity of all purchasers
- Required protection and disclosure methods in the collection of personal information
- Required reports to the Attorney General of any suspicious payments or disappearances of the regulated products
- Required training of employees with regard to the requirements of the CMEA. Retailers must self-certify as to training and compliance.
- The non-liquid dose form of regulated products may only be sold in unit dose blister packs
- Regulated products must be stored behind the counter or in a locked cabinet in such a way as to restrict public access
- Sales limits (per customer):
- Daily sales limit—must not exceed 3.6 grams of pseudoephedrine base without regard to the number of transactions
- 30-day (not monthly) sales limit—must not exceed 7.5 grams of pseudoephedrine base if sold by mail order or "mobile retail vendor"
- 30-day purchase limit—must not exceed 9 grams of pseudoephedrine base. (A misdemeanor possession offense under 21 U.S.C. § 844a for the person who buys it.)
The requirements were revised in the Methamphetamine Production Prevention Act of 2008 to require that a regulated seller of scheduled listed chemical products may not sell such a product unless the purchaser:[25]
- Presents a government issued photographic identification; and
- Signs the written logbook with their name, address, and time and date of the sale
State
Most states also have laws regulating pseudoephedrine.[74][75][76]
The states of Alabama, Arizona, Arkansas, California, Colorado, Delaware, Florida, Georgia, Hawaii (as of May 1, 2009) Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana (as of August 15, 2009), Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska,[77] Nevada, New Jersey, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia and Wisconsin have laws requiring pharmacies to sell pseudoephedrine "behind the counter". Though the drug can be purchased without a prescription, states can limit the number of units sold and can collect personal information from purchasers.[78]
The states of Oregon and Mississippi previously required a prescription for the purchase of products containing pseudoephedrine. However as of 1 January 2022 these restrictions have been repealed.[79][80] The state of Oregon reduced the number of methamphetamine lab seizures from 467 in 2004 (the final full year before implementation of the prescription only law)[81] to a new low of 12 in 2009.[82] The decrease in meth lab incidents in Oregon occurred largely before the prescription-only law took effect, according to a NAMSDL report titled Pseudoephedrine Prescription Laws in Oregon and Mississippi.[78] The report posits that the decline in meth lab incidents in both states may be due to other factors: "Mexican traffickers may have contributed to the decline in meth labs in Mississippi and Oregon (and surrounding states) as they were able to provide ample supply of equal or greater quality meth at competitive prices". Additionally, similar decreases in meth lab incidents were seen in surrounding states, according to the report, and meth-related deaths in Oregon have dramatically risen since 2007. Some municipalities in Missouri have enacted similar ordinances, including Washington,[83] Union,[84] New Haven,[85] Cape Girardeau[86] and Ozark.[87] Certain pharmacies in Terre Haute, Indiana do so as well.[88]
Another approach to controlling the drug on the state level mandated by some state governments to control the purchases of their citizens is the use of electronic tracking systems, which require the electronic submission of specified purchaser information by all retailers who sell pseudoephedrine. Thirty-two states now require the National Precursor Log Exchange (NPLEx) to be used for every pseudoephedrine and ephedrine OTC purchase, and ten of the eleven largest pharmacy chains in the US voluntarily contribute all of their similar transactions to NPLEx. These states have seen dramatic results in reducing the number of methamphetamine laboratory seizures. Prior to implementation of the system in Tennessee in 2005, methamphetamine laboratory seizures totaled 1,497 in 2004, but were reduced to 955 in 2005, and 589 in 2009.[82] Kentucky's program was implemented statewide in 2008, and since statewide implementation, the number of laboratory seizures has significantly decreased.[82] Oklahoma initially experienced success with their tracking system after implementation in 2006, as the number of seizures dropped in that year and again in 2007. In 2008, however, seizures began rising again, and have continued to rise in 2009.[82]
NPLEx appears to be successful by requiring the real-time submission of transactions, thereby enabling the relevant laws to be enforced at the point of sale. By creating a multi-state database and the ability to compare all transactions quickly, NPLEx enables pharmacies to deny purchases that would be illegal based on gram limits, age, or even to convicted meth offenders in some states. NPLEx also enforces the federal gram limits across state lines, which was impossible with state-operated systems. Access to the records is by law enforcement agencies only, through an online secure portal.[89]
Synthesis
Although pseudoephedrine occurs naturally as an alkaloid in certain plant species (for example, as a constituent of extracts from the Ephedra species, also known as ma huang, in which it occurs together with other isomers of ephedrine), the majority of pseudoephedrine produced for commercial use is derived from yeast fermentation of dextrose in the presence of benzaldehyde. In this process, specialized strains of yeast (typically a variety of Candida utilis or Saccharomyces cerevisiae) are added to large vats containing water, dextrose and the enzyme pyruvate decarboxylase (such as found in beets and other plants). After the yeast has begun fermenting the dextrose, the benzaldehyde is added to the vats, and in this environment the yeast converts the ingredients to the precursor l-phenylacetylcarbinol (L-PAC). L-PAC is then chemically converted to pseudoephedrine via reductive amination.[90]
The bulk of pseudoephedrine is produced by commercial pharmaceutical manufacturers in India and China, where economic and industrial conditions favor its mass production for export.[91]
See also
References
- ↑ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 15 August 2023.
- 1 2 3 Brunton LL, Lazo JS, Parker K, eds. (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill Medical Publishing Division. ISBN 0-07-142280-3.
- ↑ Al-Ahmad M, Hassab M, Al Ansari A (21 December 2020). "Allergic and Non-allergic Rhinitis". Textbook of Clinical Otolaryngology. Cham: Springer International Publishing. pp. 241–252. doi:10.1007/978-3-030-54088-3_22. ISBN 978-3-030-54087-6. S2CID 234142758.
Oral decongestants such as pseudoephedrine combined with an antihistamine and/or INC sprays are used in patients with nasal congestion.
- ↑ Hodges K, Hancock S, Currell K, Hamilton B, Jeukendrup AE (February 2006). "Pseudoephedrine enhances performance in 1500-m runners". Medicine and Science in Sports and Exercise. 38 (2): 329–333. doi:10.1249/01.mss.0000183201.79330.9c. PMID 16531903.
- ↑ Trinh KV, Kim J, Ritsma A (November 2015). "Effect of pseudoephedrine in sport: a systematic review". BMJ Open Sport & Exercise Medicine. 1 (1): e000066. doi:10.1136/bmjsem-2015-000066. PMC 5117033. PMID 27900142.
- ↑ Ladenburg A, Oelschlägel C (1889). "Ueber das "Pseudo-Ephedrin"" [On pseudo-ephedrine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 22 (2): 1823–1827. doi:10.1002/cber.18890220225. Archived from the original on 8 March 2021. Retrieved 19 May 2019.
- ↑ "American Urological Association – Medical Student Curriculum – Urinary Incontinence". American Urological Association. Archived from the original on 9 July 2015. Retrieved 12 August 2015.
- ↑ "Acute urinary retention due to pseudoephedrine hydrochloride in a 3-year-old child". The Turkish Journal of Pediatrics. Archived from the original on 14 August 2016. Retrieved 12 August 2015.
- ↑ Minamizawa K, Goto H, Ohi Y, Shimada Y, Terasawa K, Haji A (September 2006). "Effect of d-pseudoephedrine on cough reflex and its mode of action in guinea pigs". Journal of Pharmacological Sciences. 102 (1): 136–142. doi:10.1254/jphs.FP0060526. PMID 16974066.
- 1 2 Bicopoulos D, editor. AusDI: Drug information for the healthcare professional, 2nd edition. Castle Hill: Pharmaceutical Care Information Services; 2002.
- ↑ Weiss BD (15 January 2005). "Selecting Medications for the Treatment of Urinary Incontinence – January 15, 2005 – American Family Physician". American Family Physician. 71 (2): 315–322. Archived from the original on 7 July 2022. Retrieved 6 May 2012.
- ↑ Culligan PJ, Heit M (December 2000). "Urinary incontinence in women: evaluation and management". American Family Physician. 62 (11): 2433–44, 2447, 2452. PMID 11130230. Archived from the original on 7 September 2023. Retrieved 7 September 2023.
- 1 2 3 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Vidal C, Prieto A, Pérez-Carral C, Armisén M (April 1998). "Nonpigmenting fixed drug eruption due to pseudoephedrine". Annals of Allergy, Asthma & Immunology. 80 (4): 309–310. doi:10.1016/S1081-1206(10)62974-2. PMID 9564979.
- ↑ "Adco-Tussend". Home.intekom.com. 15 March 1993. Archived from the original on 30 April 2012. Retrieved 6 May 2012.
- ↑ Cantu C, Arauz A, Murillo-Bonilla LM, López M, Barinagarrementeria F (July 2003). "Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs". Stroke. 34 (7): 1667–1672. doi:10.1161/01.STR.0000075293.45936.FA. PMID 12791938.
- ↑ Deckx L, De Sutter AI, Guo L, Mir NA, van Driel ML (October 2016). "Nasal decongestants in monotherapy for the common cold". The Cochrane Database of Systematic Reviews. 2016 (10): CD009612. doi:10.1002/14651858.CD009612.pub2. PMC 6461189. PMID 27748955.
- 1 2 3 American Medical Association, AMA Department of Drugs (1977). AMA Drug Evaluations. PSG Publishing Co., Inc. p. 627.
- 1 2 Drug Information for the Health Care Professional. Vol. 1. Greenwood Village, Colorado.: Thomson/Micromedex. 2007. p. 2452.
- ↑ Drew CD, Knight GT, Hughes DT, Bush M (September 1978). "Comparison of the effects of D-(-)-ephedrine and L-(+)-pseudoephedrine on the cardiovascular and respiratory systems in man". British Journal of Clinical Pharmacology. 6 (3): 221–225. doi:10.1111/j.1365-2125.1978.tb04588.x. PMC 1429447. PMID 687500.
- ↑ Aljazaf K, Hale TW, Ilett KF, Hartmann PE, Mitoulas LR, Kristensen JH, et al. (July 2003). "Pseudoephedrine: effects on milk production in women and estimation of infant exposure via breastmilk". British Journal of Clinical Pharmacology. 56 (1): 18–24. doi:10.1046/j.1365-2125.2003.01822.x. PMC 1884328. PMID 12848771.
- 1 2 Hatton RC, Winterstein AG, McKelvey RP, Shuster J, Hendeles L (March 2007). "Efficacy and safety of oral phenylephrine: systematic review and meta-analysis". The Annals of Pharmacotherapy. 41 (3): 381–390. doi:10.1345/aph.1H679. PMID 17264159. S2CID 25627664.
- 1 2 "Legal Requirements for the Sale and Purchase of Drug Products Containing Pseudoephedrine, Ephedrine, and Phenylpropanolamine". U.S. Food and Drug Administration. 9 March 2006. Archived from the original on 25 December 2023. Retrieved 7 September 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 "CMEA (The Combat Methamphetamine Epidemic Act of 2005)". DEA Diversion Control Division. 14 July 2017. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 "2011 – Final Rule: Implementation of the Methamphetamine Production Prevention Act of 2008". DEA Diversion Control Division. 1 November 2011. Archived from the original on 17 February 2020. Retrieved 17 February 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Pseudoephedrine Compliance". Walgreens. Archived from the original on 30 January 2016. Retrieved 24 January 2016.
- ↑ "Target Announces That All Products Containing Pseudoephedrine Will Be Placed Behind Pharmacy Counter". PRNewswire. Archived from the original on 31 January 2016. Retrieved 24 January 2016.
- ↑ "Microsoft Word - RedListE2007.doc" (PDF). Archived from the original (PDF) on 27 February 2008. Retrieved 6 May 2012.
- ↑ "WADA 2010 Prohibited List Now Published – World Anti-Doping Agency". Wada-ama.org. Archived from the original on 20 February 2012. Retrieved 6 May 2012.
- ↑ "Elimination of ephedrines in urine following multiple dosing the consequences for athletes in relation to doping control". Archived from the original on 29 August 2021. Retrieved 24 February 2014.
- ↑ Strano-Rossi S, Leone D, de la Torre X, Botrè F (August 2009). "The relevance of the urinary concentration of ephedrines in anti-doping analysis: determination of pseudoephedrine, cathine, and ephedrine after administration of over-the-counter medicaments". Therapeutic Drug Monitoring. 31 (4): 520–526. doi:10.1097/FTD.0b013e3181ac6006. PMID 19571776. S2CID 21333203.
- ↑ "Ressources" (PDF). Archived from the original (PDF) on 29 May 2014. Retrieved 18 August 2016.
- ↑ Jolley D, Dawson B, Maloney SK, White J, Goodman C, Peeling P (June 2014). "Hydration and urinary pseudoephedrine levels after a simulated team game". International Journal of Sport Nutrition and Exercise Metabolism. 24 (3): 325–332. doi:10.1123/ijsnem.2013-0076. PMID 24458099.
- ↑ "Silken tests positive". Archived from the original on 4 March 2014. Retrieved 24 February 2014.
- ↑ Wallechinsky D (2009). Complete Book of the Winter Olympics. ISBN 9781553655022. Retrieved 9 July 2010.
- ↑ "2000 World Championships – Pairs". Ice Skating International. Archived from the original on 14 November 2011. Retrieved 6 June 2010.
- ↑ "Summer Olympics 2000 Raducan tests positive for stimulant". Assets.espn.go.com. 26 September 2000. Archived from the original on 2 October 2011. Retrieved 6 May 2012.
- ↑ "Amanar Tops Romanian Money List". International Gymnast Magazine Online. 15 October 2000. Archived from the original on 15 July 2001. Retrieved 6 May 2012.
- ↑ "IOC issues a reprimand against Slovakian ice hockey player". Archived from the original on 8 March 2014. Retrieved 24 February 2014.
- ↑ "Sweden's Bäckström tests positive for banned substance". Archived from the original on 27 February 2014. Retrieved 24 February 2014.
- ↑ "IOC Decision – Swedish ice hockey player Nicklas Backstrom to receive Sochi silver medal". IOC. 14 March 2014. Archived from the original on 15 March 2014. Retrieved 14 March 2014.
- ↑ Prewitt A (15 January 2015). "Nicklas Backstrom's Olympic doping appeal resolved with reprimand". The Washington Post. Archived from the original on 17 January 2015. Retrieved 18 January 2015.
- ↑ Boland DM, Rein J, Lew EO, Hearn WL (October 2003). "Fatal cold medication intoxication in an infant". Journal of Analytical Toxicology. 27 (7): 523–526. doi:10.1093/jat/27.7.523. PMID 14607011.
- ↑ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, California: Biomedical Publications. pp. 1344–1346. Archived from the original on 4 December 2020. Retrieved 19 August 2010.
- ↑ Patil PN, Tye A, Lapidus JB (May 1965). "A Pharmacological Study of the Ephedrine Isomers". The Journal of Pharmacology and Experimental Therapeutics. 148 (2): 158–168. PMID 14301006. Archived from the original on 7 January 2020. Retrieved 24 December 2009.
- ↑ Martindale (1989). Reynolds JEF (ed.). Martindale: The complete drug reference (29th ed.). London: Pharmaceutical Press. ISBN 0-85369-210-6.
- ↑ Proposed International Non-Proprietary Names (Prop. I.N.N.): List 11 WHO Chronicle, Vol. 15, No. 8, August 1961, pp. 314–20
- ↑ "Benadryl Allergy Relief Plus Decongestant Capsules - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Datapharm. Archived from the original on 22 November 2023. Retrieved 22 November 2023.
- ↑ "Drug Pamphlet: Congestal". 12 February 2017. Archived from the original on 13 December 2018. Retrieved 13 December 2018.
- ↑ "Photographic image of Congestal tablets information sheet" (JPG). 1.bp.blogspot.com. Archived from the original on 13 December 2018. Retrieved 28 February 2022.
- ↑ "Project STOP video – Online Advertising". Innovationrx.com.au. Archived from the original on 26 April 2012. Retrieved 6 May 2012.
- ↑ Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Yao R, Staudinger H, et al. (February 2009). "A placebo-controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber". Annals of Allergy, Asthma & Immunology. 102 (2): 116–120. doi:10.1016/S1081-1206(10)60240-2. PMID 19230461.
- ↑ Eccles R (January 2007). "Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse". British Journal of Clinical Pharmacology. 63 (1): 10–14. doi:10.1111/j.1365-2125.2006.02833.x. PMC 2000711. PMID 17116124.
- ↑ "PRAC February 2023 – Start of a safety review of pseudoephedrine-containing medicines | FAMHP". www.famhp.be. Archived from the original on 15 August 2023. Retrieved 15 August 2023.
- ↑ "Archived – Health Canada Reminds Canadians not to use Ephedra/Ephedrine Products". healthycanadians.gc.ca. 20 August 2021. Archived from the original on 27 April 2014. Retrieved 1 July 2015.
- ↑ "What ingredients are commonly found in OTC cough and cold products? What do they do?". Pharmacy Technician's Letter. Archived from the original on 25 October 2015. Retrieved 25 August 2015.
- ↑ "Family Health Online – Family Health Magazine -PHARMACY CARE – Over-the-Counter Medication – Why must I ask for that?". Archived from the original on 18 June 2016. Retrieved 18 August 2016.
- ↑ "Gobierno prohĂbe antigripales con pseudoefedrina a partir de finales de 2010 – Archivo – Archivo Digital de Noticias de Colombia y el Mundo desde 1.990" [Government Prohibits Flu Pseudoephedrine in Late 2010 – Archive – News Archive Digital Colombia and the World since 1990] (in Spanish). Eltiempo.com. 8 July 2009 [Published 11 August 2009]. Archived from the original on 21 May 2011. Retrieved 6 May 2012.
- ↑ "Keskmise eestlase kodust leiab portsu dopingut". Uudised (in Estonian). 28 October 2016. Archived from the original on 9 July 2023. Retrieved 9 July 2023.
- ↑ "Kysy Kiminkiseltä: Parasta flunssan hoitoa on lepo ja mummon mustaviinimarjamehu". www.apu.fi. 29 January 2018. Archived from the original on 9 July 2023. Retrieved 9 July 2023.
- ↑ "Customs Information". Consulate-General of Japan in Seattle. Archived from the original on 19 August 2015. Retrieved 27 August 2015.
- ↑ "Prohibirán definitivamente uso de pseudoefedrina" [A Permanent Ban on Pseudoephedrine] (in Spanish). 23 November 2007 [Published 2 December 2007]. Archived from the original on 19 December 2013. Retrieved 17 December 2013.
- ↑ Megget K (2 September 2007). "Pseudoephedrine drugs still OTC". Archived from the original on 29 August 2021. Retrieved 15 October 2018.
- ↑ "Controlled drugs". Health.govt.nz. Archived from the original on 8 July 2017. Retrieved 6 July 2017.
- ↑ "Misuse of Drugs Act 1975 No 116, Public Act 8 Exemptions from sections 6 and 7". Legislation.govt.nz. 22 December 2016. Archived from the original on 15 July 2017. Retrieved 6 July 2017.
- ↑ "Ephedrine and Pseudoephedrine to Become Controlled Drugs". Medsafe, New Zealand Medicines and Medical Devices Safety Authority. Archived from the original on 8 March 2007.
- ↑ "Chemical Brothers". Listener (New Zealand). Archived from the original on 23 May 2010.
- ↑ "Misuse of Drugs Amendment Bill". parliament.nz. Archived from the original on 6 July 2017. Retrieved 6 July 2017.
- ↑ "Kontrole Tabi İlaçlar" (PDF). Social Security Institution of Republic of Turkey. July 2013. Archived from the original (PDF) on 11 March 2014. Retrieved 11 March 2014.
- ↑ "Drug Safety Update, March 2008". The Medicines and Healthcare products Regulatory Agency and the Commission on Human Medicines. 30 June 2008. Archived from the original (PDF) on 14 June 2011.
- ↑ "Search OregonLive.com". The Oregonian. Archived from the original on 17 August 2016. Retrieved 13 December 2010.
- ↑ "How Legislation Changed Meth Purity" (PDF). The Oregonian. Archived from the original (PDF) on 15 November 2011. Retrieved 13 December 2010.
- 1 2 "DEA Interim Final Regulation: Ephedrine, Pseudoephedrine, and Phenylpropanolamine Requirements" (PDF). Archived from the original (PDF) on 18 October 2006.
- ↑ "State Ephedrine and Pseudoephedrine Single Over-The-Counter Transaction Limits". Namsdl.org. National Alliance for Model State Drug Laws (NAMSDL). 2013. Archived from the original (PDF) on 14 April 2016. Retrieved 3 December 2016.
- ↑ "State Daily Gram Limits for Over-The-Counter Transactions Involving Ephedrine and Pseudoephedrine". Namsdl.org. National Alliance for Model State Drug Laws (NAMSDL). 2013. Archived from the original (PDF) on 14 August 2016. Retrieved 3 December 2016.
- ↑ "State 30 Day Gram Limits for Over-The-Counter Transactions Involving Ephedrine and Pseudoephedrine". Namsdl.org. National Alliance for Model State Drug Laws (NAMSDL). 2013. Archived from the original (PDF) on 15 August 2016. Retrieved 3 December 2016.
- ↑ "Nebraskans to sign for Sudafed". Lincoln Journal-Star. 13 March 2006. Archived from the original on 22 June 2018. Retrieved 20 August 2012.
- 1 2 "The National Alliance for Model State Drug Laws (NAMSDL) – Issues and Events". www.namsdl.org. Archived from the original on 3 October 2015. Retrieved 24 October 2015.
- ↑ "MS Senate passes bill to restrict pseudoephedrine sales". Associated Content. WLOX. 2 February 2010. Archived from the original on 28 September 2011. Retrieved 8 May 2010.
- ↑ Bovett R (15 November 2010). "How to Kill the Meth Monster". The New York Times. Archived from the original on 1 May 2011. Retrieved 16 November 2010.
- ↑ "Archived copy". Archived from the original on 9 March 2007. Retrieved 20 January 2007.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 "DEA, Maps of Methamphetamine Lab Incidents". Justice.gov. Archived from the original on 10 September 2012. Retrieved 6 May 2012.
- ↑ "Council Passes Law Restricting Pseudoephedrine". The Washington Missourian. 7 July 2009. Archived from the original on 12 March 2012. Retrieved 10 December 2010.
- ↑ "Union Board Approves Pseudoephedrine Ordinance". The Washington Missourian. 13 October 2009. Archived from the original on 12 March 2012. Retrieved 10 December 2010.
- ↑ "New Haven Passes Prescription Law". The Washington Missourian. 11 November 2010. Archived from the original on 12 March 2012. Retrieved 10 December 2010.
- ↑ "Cape Girardeau City Council passes prescription requirement for pseudoephedrine". The Southeast Missourian. 7 December 2010. Archived from the original on 28 January 2011. Retrieved 10 December 2010.
- ↑ "Ozark passes pseudoephedrine ban: Drug now prescription-only". CCHeadliner.com. 18 June 2013. Archived from the original on 24 February 2021. Retrieved 17 December 2013.
- ↑ Trigg L (20 May 2010). "Four Valley pharmacies to require prescriptions for certain products to help fight meth problem". Terre Haute Tribune-Star. Archived from the original on 24 May 2010. Retrieved 28 May 2010. (Subscription required, free access for first 30 days)
- ↑ "NPLEx – National Precursor Log Exchange". www.nplexservice.com. Archived from the original on 12 October 2015. Retrieved 24 October 2015.
- ↑ Oliver AL, Anderson BN, Roddick FA (1999). "Factors affecting the production of L-phenylacetylcarbinol by yeast: a case study". Advances in Microbial Physiology. Vol. 41. pp. 1–45. doi:10.1016/S0065-2911(08)60164-2. ISBN 978-0-12-027741-4. PMID 10500843.
- ↑ "Clamp down on shipments of raw ingredients, Oregonian, Press, Congressional Caucus to Fight and Control Methamphetamine". U.S. House of Representatives. 31 August 2005. Archived from the original on 9 May 2006. Retrieved 7 September 2023.