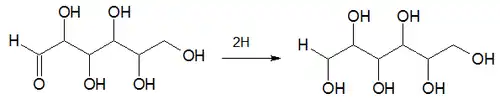
The Creighton process involves the hydrogenation of a 6 carbon chain aldehyde.[1][2] The reactant is 2,3,4,5,6-pentahydroxyhexanal (an aldehyde) and the product is 1,2,3,4,5,6-hexanehexol (an alcohol). The product thus has two more hydrogen atoms than the reactant: -CHO is replaced by -CH2OH.
The Creighton process was patented in the 1920s.[3]
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.