 | |
| Clinical data | |
|---|---|
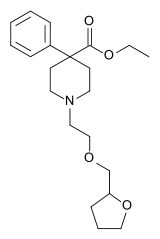
| Other names | ethyl 4-phenyl-1-(2-tetrahydrofurfuryloxyethyl)piperidine-4-carboxylate |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.017.451 |
| Chemical and physical data | |
| Formula | C21H31NO4 |
| Molar mass | 361.482 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Furethidine[2][3][4] is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine),[5] but with around 25x higher potency.[6] According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine (table VII).[7][8]
Furethidine is not currently used in medicine and is a Class A/Schedule I drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as analgesia, sedation, nausea and respiratory depression.[9] In the United States it is a Schedule I Narcotic controlled substance with the ACSCN of 9626.[10]
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ GB 797448, Frearson PM, Stern ES, "Novel piperidine compounds and their production", published 2 July 1958, assigned to J F Macfarlan & Co Ltd
- ↑ Frearson Peter Marshall; Stern Edward Severin, DE 1256219 (1967 to Glaxo Lab Ltd).
- ↑ Frearson, P. M., Hardy, D. G., Stern, E. S. (1960). "426. Some new analogues of pethidine. Part IV. Substituents at the 1-position incorporating cyclic ether groups". Journal of the Chemical Society (Resumed): 2103. doi:10.1039/jr9600002103. ISSN 0368-1769.
- ↑ Maul C, Buschmann H, Sundermann B (2005). "Opioids: 3.3 Synthetic Opioids.". Analgesics. Wiley-VCH. pp. 159–169. ISBN 978-3-527-30403-5.
- ↑ Casy AF, Parfitt RY. Opioid analgesics, chemistry and receptors. 1986, Plenum Press, New York. pp 234-235. ISBN 0-306-42130-5
- ↑ Blair, A. M. J. N.; Stephenson, R. P. (1960). "ANALGESIC ACTION OF ETHYL 4-PHENYLPIPERIDINE-4-CARBOXYLATES WITH OXYGENATED 1-SUBSTITUENTS". British Journal of Pharmacology and Chemotherapy. 15 (2): 247–253. doi:10.1111/j.1476-5381.1960.tb01239.x.
- ↑ Lister, R. E. (1960). "PHARMACOLOGICAL ACTIONS OF TWO NEW PETHIDINE ANALOGUES". British Journal of Pharmacology and Chemotherapy. 15 (2): 254–259. doi:10.1111/j.1476-5381.1960.tb01240.x.
- ↑ Cahal DA, Dare JG, Keith D (February 1961). "A sequential trial of analgesics in labour". The Journal of Obstetrics and Gynaecology of the British Commonwealth. 68: 88–93. doi:10.1111/j.1471-0528.1961.tb02689.x. PMID 13689779. S2CID 27397119.
- ↑ "Controlled Substances - Alphabetical Order" (PDF). Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice. 2020. p. 9.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.