 | |
 | |
| Names | |
|---|---|
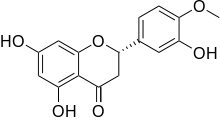
| IUPAC name
(2S)-3′,5,7-Trihydroxy-4′-methoxyflavan-4-one | |
| Systematic IUPAC name
(2S)-5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.538 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.282 g·mol−1 |
| Melting point | 226–228 °C (439–442 °F; 499–501 K) |
| Solubility in other solvents | Sol. EtOH, alkalis |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hesperetin is the 4'-methoxy derivative of eriodictyol, a flavanone. Hesperetin's 7-O-glycoside, hesperidin, is a naturally occurring flavanon-glycoside, the main flavonoid in lemons and sweet oranges.[1] Hesperetin (and naringenin, the parent flavanone of naringin) are not found to a significant extent in Citrus spp.[2]
Glycosides
A variety of glycosides of hesperetin are known, including:
- Hesperidin (hesperetin-7-O-rutinoside) is a water-insoluble flavonoid glycoside whose solubility is below 5 μg/ml in water.[3] Hesperidin is found in citrus fruits and upon ingestion it releases its aglycone, hesperetin.
- Neohesperidin is the 7-O-neohesperidoside of hesperetin.
- Hesperetin-7-O-α-L-Rhamnopyranoside (CAS 66513-83-5) is found in the roots of clammy cherry[4] (Cordia obliqua a.k.a. Cordia obliqua var. wallichii[5]).
Metabolism
Hesperidin 6-O-α-L-rhamnosyl-β-D-glucosidase is an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose. It is found in the hyphomycetes species Stilbella fimetaria.
Effects
Hesperetin was found to be affecting the slow inactivation phase of inward sodium current channels (INa) and therefore could be used as a template to develop drugs against lethal cardiac arrhythmias in LQT3.[6] Hesperetin also inhibits TRPM3 channels.[7]
References
- ↑ "Hesperetin".
- ↑ Lewinsohn, E; Britsch, L; Mazur, Y; Gressel, J (1989). "Flavanone Glycoside Biosynthesis in Citrus: Chalcone Synthase, UDP-Glucose:Flavanone-7-O-Glucosyl-Transferase and -Rhamnosyl-Transferase Activities in Cell-Free Extracts". Plant Physiology. 91 (4): 1323–1328. doi:10.1104/pp.91.4.1323. PMC 1062186. PMID 16667183.
- ↑ Majumdar S.; Srirangam, R. (2009). "Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid". Pharm. Res. 26 (5): 1217–1225. doi:10.1007/s11095-008-9729-6. PMC 2664388. PMID 18810327.
- ↑ http://ccd.chemnetbase.com/AAA00.entry?parentCHNumber=CNB06-R:CNB07-S%5B%5D
- ↑ http://www.newcropslisting.info/listing/species_pages_C/Cordia_obliqua.htm%5B%5D
- ↑ Alvarez‐Collazo, Julio; López‐Requena, Alejandro; Galán, Loipa; Talavera, Ariel; Alvarez, Julio L.; Talavera, Karel (27 March 2019). "The citrus flavanone hesperetin preferentially inhibits slow‐inactivating currents of a long QT syndrome type 3 syndrome Na+ channel mutation". British Journal of Pharmacology. 176 (8): 1090–1105. doi:10.1111/bph.14577. PMC 6451064. PMID 30650182.
- ↑ Straub, Isabelle; Krügel, Ute; Mohr, Florian; Teichert, Jens; Rizun, Oleksandr; Konrad, Maik; Oberwinkler, Johannes; Schaefer, Michael (November 2013). "Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo". Molecular Pharmacology. 84 (5): 736–750. doi:10.1124/mol.113.086843. ISSN 1521-0111. PMID 24006495.
External links
 Media related to Hesperetin at Wikimedia Commons
Media related to Hesperetin at Wikimedia Commons