 | |
 | |
| Names | |
|---|---|
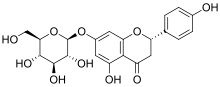
| IUPAC name
(2S)-7-(β-D-Glucopyranosyloxy)-4′,5-dihydroxyflavan-4-one | |
| Systematic IUPAC name
(2S)-5-Hydroxy-2-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Naringenin-7-O-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.696 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H22O10 | |
| Molar mass | 434.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prunin is a flavanone glycoside found in immature citrus fruits[1][2] and in tomatoes.[3] Its aglycone form is called naringenin.
Metabolism
Glucosidase breaks prunin into glucose and naringenin.
References
- ↑ Berhow, Mark A.; Vandercook, Carl E. (1989). "Biosynthesis of naringin and prunin in detached grapefruit". Phytochemistry. 28 (6): 1627–1630. doi:10.1016/S0031-9422(00)97813-0. ISSN 0031-9422.
- ↑ Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid hromatography/electrospray ionization tandem mass spectrometry. Anna Vallverdu´-Queralt, Olga Jauregui, Alexander Medina-Remon, Cristina Andres-Lacueva and Rosa M. Lamuela-Raventos, Rapid Commun. Mass Spectrom., 2010, volume 24, pages 2986–2992, doi:10.1002/rcm.4731
Bibliography
- Habelt, Konrad; Pittner, Fritz (1983). "A rapid method for the determination of naringin, prunin, and naringenin applied to the assay of naringinase". Analytical Biochemistry. 134 (2): 393–397. doi:10.1016/0003-2697(83)90314-7. ISSN 0003-2697. PMID 6418025.
External links
 Media related to Prunin at Wikimedia Commons
Media related to Prunin at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.