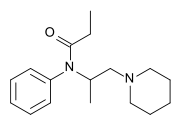
 | |
| Clinical data | |
|---|---|
| Routes of administration | ? |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.517 |
| Chemical and physical data | |
| Formula | C17H26N2O |
| Molar mass | 274.408 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Phenampromide[2] is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and diampromide. It was invented in the 1960s[3] by American Cyanamid Co. Although never given a general release, it was trialled and 50 mg codeine ≈ 60 mg phenampromide. Tests on the 2 isomers showed that all of the analgesic effects were caused by the (S) isomer. Introduction of a phenyl group to the 4-position of the piperidine-ring produces a drug 60-fold more potent than morphine.[4] The most potent reported derivative is 4-hydroxy-4-phenyl phenapromide which displays analgesic activity some x150 greater than morphine.[5]
Phenampromide produces similar effects to fentanyl, including analgesia, sedation, dizziness and nausea.
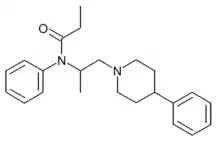
Phenampromide is in Schedule I of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9638 with a zero aggregate manufacturing quota as of 2014. The free base conversion ratio for salts includes 0.88 for the hydrochloride.[6] It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as fentanyl.
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ US3016382A N-substituted anilides and method of preparing the same
- ↑ Portoghese PS (March 1965). "Stereochemical Studies on Medicinal Agents II. Absolute Configuration of (-)-Phenampromide". Journal of Medicinal Chemistry. 8: 147–50. doi:10.1021/jm00326a001. PMID 14332652.
- ↑ Lenz GR, Evans SM, Walters DE, Hopfinger AJ (1986). Opiates. Orlando: Academic Press. ISBN 978-0-12-443830-9.
- ↑ Casy AF, Parfitt RT (1986). Opiate Aalgesics Chemistry and Receptors. New York: Springer Science+ Business Media. ISBN 978-1-4899-0587-1.
- ↑ "Quotas - 2014". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.