 | |
 | |
| Clinical data | |
|---|---|
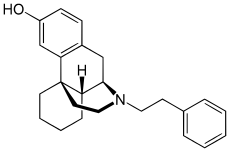
| Other names | (-)-3-hydroxy- N- (2-phenylethyl) morphinan |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.732 |
| Chemical and physical data | |
| Formula | C24H29NO |
| Molar mass | 347.502 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phenomorphan[2] is an opioid analgesic. It is not currently used in medicine, but has similar side-effects to other opiates, which include itching, nausea and respiratory depression.
Phenomorphan is a highly potent drug due to the N-phenethyl group, which boosts affinity to the μ-opioid receptor, and so phenomorphan is around 10x more potent than levorphanol, which is itself 6-8x the potency of morphine. Other analogues where the N-(2-phenylethyl) group has been replaced by other aromatic rings[3] are even more potent, with the N-(2-(2-furyl)ethyl) and the N-(2-(2-thienyl)ethyl) analogues being 60x and 45x stronger than levorphanol, respectively.[4]
See also
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ US patent 2885401, Grussner A, Hellerbach J, Schnider O, "Process for making morphinan derivatives and products available thereby", published 1956-03-22, issued 1959-05-05
- ↑ US patent 2970147, Andre Grussner, Joseph Hellerbach, Otto Schnider, "3-hydroxy-N-(heterocyclic-ethyl)-morphinans", published 1958-11-26, issued 1961-01-31
- ↑ Hellerbach J, Schnider O, Besendorf H, Pellmont B (1966). Synthetic Analgesics: Part IIA. Morphinans. International series of monographs on organic chemistry. Pergamon Press.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.