| |
| Clinical data | |
|---|---|
| Trade names | Torecan, Norzine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.381 |
| Chemical and physical data | |
| Formula | C22H29N3S2 |
| Molar mass | 399.62 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Thiethylperazine (Torecan, Norzine) is an antiemetic[1] of the phenothiazine class. It is an antagonist of dopamine receptors (DRD1, DRD2, DRD4) as well as of 5-HT2A, 5-HT2C receptors, mAChRs (1 through 5), α1 adrenergic receptor and H1 receptor.
Thiethylperazine activates the transport protein ABCC1 that clears beta-amyloid from brains of mice.[2]
Pharmacokinetics
Distribution
This drug is highly lipofilic and it binds with membranes and serum proteins (over 85%). It accumulates in organs with high blood flow and penetrates the placenta. It cannot be removed with dialysis.
Metabolism
It is mainly metabolised in the liver and only 3% is eliminated unchanged. Torecan's half-life is 12 h.
Teratogenicity
In toxic doses above the terapeutic window, it increases the rate of cleft palate occurrence.
Antipsychotic activity
Theithylperazine may possess antipsychotic activity[4] due to the antagonism of 5-HT2 and D2 receptors. It can cause extrapyramidal symptoms. Nevertheless, it was never marketed as an antipsychotic.
One cause of acute dystonia occurred in a 19-year-old male patient after discontinuation of this drug.[5]
Overdose
Signs of acute thiethylperazine overdose include: extrapyramidal symptoms, confusion, convulsions, respiratory depression and hypotension.
Synthesis
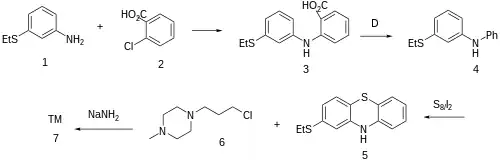
Goldberg reaction between 3-(ethylsulfanyl)aniline [1783-82-0] (1) and 2-chlorobenzoic acid [118-91-2] (2) to give the diarylamine, CID:82254530 (3). The carboxyl in the anthranilic acid residue, having performed its activating function, is then thermolytically removed to form [68083-49-8] (4). Upon treatment with sulfur and iodine, we get predominantly the phenothiazine [46815-10-5] (5); The rxn may well be aided by the presence of the electron donating thioether at the para-position. Alkylation with 1-(ɣ̞-chloropropyl)-4-methylpiperazine [104-16-5] (6) in the presence of sodamide affords Thiethylperazine (7).
References
- ↑ Tamboline BL, Mcgillivray DC, Bogoch A (February 1965). "The Effects of Thiethylperazine Dimaleate (Torecan) on Nausea and Vomiting". Canadian Medical Association Journal. 92 (8): 422–423. PMC 1928133. PMID 14261157.
- ↑ Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, et al. (October 2011). "Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice". The Journal of Clinical Investigation. 121 (10): 3924–3931. doi:10.1172/JCI57867. PMC 3195473. PMID 21881209.
- "Alzheimer disease: Transport protein ABCC1 plays key role in clearing beta-amyloid from brains of mice". ScienceDaily (Press release). September 1, 2011.
- ↑ "Charakterystyka Produktu Leczniczego". rejestrymedyczne.ezdrowie.gov.pl (in Polish). Archived from the original on October 20, 2022. Retrieved 14 May 2023.
- ↑ Rotrosen J, Angrist BM, Gershon S, Aronson M, Gruen P, Sachar EJ, et al. (September 1978). "Thiethylperazine; clinical antipsychotic efficacy and correlation with potency in predictive systems". Archives of General Psychiatry. 35 (9): 1112–1118. doi:10.1001/archpsyc.1978.01770330086008. PMID 99115.
- ↑ Khanderia U (July 1985). "Recurrent dystonic reactions induced by thiethylperazine". Drug Intelligence & Clinical Pharmacy. 19 (7–8): 550–551. doi:10.1177/106002808501900708. PMID 4028959. S2CID 44453678.
- ↑ Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, et al. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 1. Mitteilung. Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1061–1072. doi:10.1002/hlca.19580410419.
- ↑ Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, et al. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1072–1108. doi:10.1002/hlca.19580410420.
- ↑ US 3336197, Jany R, Bourquin jP, Gamboni G, Schwarb G, issued 1967, assigned to Sandoz KK.