 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Deparon, Tinoran |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 35 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
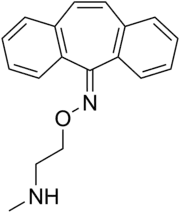
| Formula | C18H18N2O |
| Molar mass | 278.355 g·mol−1 |
| 3D model (JSmol) | |
| |
Demexiptiline (brand names Deparon, Tinoran) is a tricyclic antidepressant (TCA) used in France for the treatment of depression.[2][3] It acts primarily as a norepinephrine reuptake inhibitor similarly to desipramine.[4]
Synthesis
The synthesis is similar to that reported for Noxiptiline but also differs in a couple of respects.
The condensation between Dibenzosuberenone [2222-33-5] (1) and hydroxylamine (2) gives the oxime, [1021-91-6] (3). Base catalyzed attachment of the sidechain by reaction with 2-Chloro-N-Methylethanamine [32315-92-7] (4) completed the synthesis of demexiptiline (5).
References
- ↑ Dörwald FZ (4 February 2013). Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. John Wiley & Sons. pp. 313–. ISBN 978-3-527-64565-7.
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. p. 301. ISBN 3-88763-075-0.
- ↑ Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. p. 583. ISBN 0-412-46630-9.
- ↑ Teste JF, Pelsy-Johann I, Decelle T, Boulu RG (1993). "Anti-immobility activity of different antidepressant drugs using the tail suspension test in normal or reserpinized mice". Fundamental & Clinical Pharmacology. 7 (5): 219–26. doi:10.1111/j.1472-8206.1993.tb00235.x. PMID 8370568. S2CID 24240307.
- ↑ Aichinger G, Behner O, Hoffmeister F, Schütz S (June 1969). "[Basic tricyclic oxyiminoethers and their pharmacological properties]". Arzneimittel-Forschung. 19: Suppl 5a:838+. PMID 5819763.
- ↑ US 3989844, Schütz S, Behner O, Hoffmeister F, issued 1976, assigned to Bayer AG. & U.S. Patent 3,963,778
- ↑ DE 1247302, Schütz S, Behner O, Hoffmeister F, issued 1967, assigned to Bayer AG.
Further reading
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Classes | |
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
