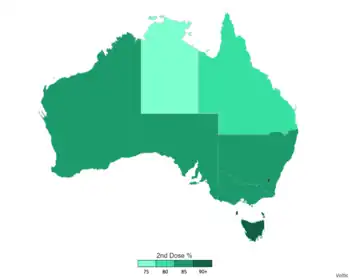
 COVID-19 vaccinated map of Australia (as of 6 August 2022)
[1] | |
| Date | 22 February 2021 – 13 April 2023 |
|---|---|
| Location | |
| Cause | COVID-19 pandemic in Australia |
| Target | Immunisation of Australians against COVID-19 |
| Budget | A$1.87 billion[2] |
| Participants |
|
| Outcome | 97% of the eligible Australian population aged 12+ have received one dose 95.2% of the eligible Australian population aged 12+ are fully vaccinated 64.5% of the eligible Australian population aged 12+ are booster given |
| Website | Department of Health and Aged Care |


The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers[lower-alpha 1] and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August.[4][5][6][7] Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.[8]
As of 3 August 2022, Australia had administered 62,492,656 vaccine doses across the country.[3][9][10] The country's vaccination rollout initially faced criticism for its slow pace and late start, falling far below initial government targets.[11][12] Despite this, Australia began vaccinating its citizens at a comparatively fast pace, overtaking the United States in first dose coverage by 10 October 2021.[13] Over 95% of the Australian population aged 12 and over are now fully vaccinated.[14][15]
Vaccine rollout and distribution

The federal government has stated it will provide free COVID-19 vaccinations to everyone living in Australia, largely regardless of immigration status. Like most vaccines, Australians do not need a prescription to receive them.[16]
COVID-19 vaccine national rollout phases
| Order | Priority group | Number of eligible (estimated) [18] | Number of doses targeted [lower-alpha 2] | Progress [lower-alpha 3] |
|---|---|---|---|---|
| Phase 1a | ||||
| 1 | Quarantine, border & front-line health care workers | 678,000 | up to 1.4 million | Completed |
| 2 | Front-line health care worker sub-groups for prioritisation | |||
| 3 | Aged care and disability care staff | |||
| 4 | Aged care and disability care residents | |||
| Phase 1b | ||||
| 5 | Elderly adults aged 80 years and over | 6,139,000 | up to 14.8 million | Completed |
| 6 | Elderly adults aged 70–79 years | |||
| 7 | Other health care workers | |||
| 8 | Aboriginal and Torres Strait Islander people aged 55 and over | |||
| 9 | Adults with an underlying medical condition, including those with a disability | |||
| 10 | Critical and high-risk workers, including defence, emergency services and meat processing | |||
| Phase 2a | ||||
| 11 | Elderly adults aged 60–69 years | 6,570,000 | up to 15.8 million | Completed |
| 12 | Adults aged 40–59 years | |||
| 13 | Aboriginal and Torres Strait Islander people aged 18–54 | |||
| 14 | Other critical and high-risk workers | |||
| Phase 2b | ||||
| 15 | Adults aged 16–39 years | 6,643,000 | up to 16 million | Completed |
| 16 | Any unvaccinated Australians from previous phases | |||
| Phase 3 | ||||
| 17 | Australians aged 12–15 years | 1,243,990 | up to 2.4 million | Completed |
| Phase 4 | ||||
| 18 | Booster dose for immunocompromised | 500,000 | 500,000 | Completed |
| 19 | Booster dose for aged 18+ | 20,037,617 | 20 million | Completed |
| 20 | Children aged 5–11 years | - | - | Completed |
On 21 February 2021, a day before the previously announced program start date, Prime Minister Scott Morrison, Chief Medical Officer Paul Kelly, Chief Nurse Alison McMillan, Kris Matthews and "a small group" of aged care staff and residents became the first Australians to receive the Pfizer–BioNTech vaccine. The early vaccination was heavily televised with the hopes of reassuring Australians about the quality, efficacy, and safety of COVID-19 vaccines.[19]
On 22 March, Health Minister Greg Hunt announced the start of the phase-1b vaccination roll-out. In this phase, more than 6 million Australians are targeted for inoculation, and approximate 1,000 GP clinics are participating in vaccination all over the nation to ramp up the speed of vaccination.[20]
The Federal Government of Australia has decided to prioritise people 50 years or older for vaccination. They will be eligible for vaccination from 3 May 2021 at General Practice Respiratory Clinics and state or territory vaccination clinics. From 17 May, people over 50 can also get their vaccination at selected participating GP clinics. The Australian Technical Advisory Group on Immunisation advised the government to reserve the Pfizer vaccine for those under 50 years of age, and the AstraZeneca vaccine will be administered for phase 2a.[17]
On 19 August 2021, an announcement was made by Prime Minister Scott Morrison that adult residents aged 16–39 will be eligible for the Pfizer vaccine from 30 August 2021.[21]
On 5 December 2021, the Therapeutic Goods Administration, Australia's medical regulator, approved access for five to 11-year-olds to the Pfizer vaccine.[22] As of 10 December 2021, it was planned to start vaccinating children aged 5 to 11 with the Pfizer vaccine from 10 January 2022.[23]
Vaccination statistics
Cumulative vaccinations in Australia
Includes population aged 12+ | ||||||
Daily vaccinations chart of Australia
Cumulative vaccinations in states/territories
Includes population age 12+ | ||||||
Vaccination rollout by state and territory
| State or territory | Eligible population aged 12+ [24] | First dose administered | Second dose administered | Population received first dose (12+) | Population received both doses (12+) | Ref. |
|---|---|---|---|---|---|---|
| Australian Capital Territory | 363,730 | 365,858 | 358,999 | >99% | 99% | [9][25] |
| New South Wales | 6,955,981 | 6,535,213 | 6,405,004 | 94% | 92% | [26][9][25] |
| Northern Territory | 203,631 | 177,480 | 159,560 | 87% | 78% | [9][25] |
| Queensland | 4,382,853 | 3,784,644 | 3,419,829 | 86% | 78% | [27][9][25] |
| South Australia | 1,523,147 | 1,363,344 | 1,246,285 | 90% | 82% | [28][9][25] |
| Tasmania | 466,480 | 438,183 | 408,599 | 94% | 88% | [29][9][25] |
| Victoria | 5,716,185 | 5,325,153 | 5,193,385 | 93% | 91% | [30][9][25] |
| Western Australia | 2,247,847 | 1,937,765 | 1,724,451 | 86% | 77% | [31][9][25] |
| 21,863,949 | 20,142,711 | 19,101,361 | 92% | 87% | [32] |
Vaccination statistics for all age groups
| Population age group | Partially vaccinated | Fully Vaccinated |
|---|---|---|
| 0+ | 78.4% | 74.3% |
| 12+ | 92.1% | 87.4% |
| 16+ | 93.0% | 88.5% |
| 70+ | >99% | 98.3% |
Vaccination doses by jurisdiction and delivery channel
| State or territory | Vaccination hub | Aged care | GP clinics |
|---|---|---|---|
| Australian Capital Territory | 730,672 | 16,549 | 678,151 |
| New South Wales | 5,002,549 | 557,944 | 11,715,218 |
| Northern Territory | 316,951 | 7,406 | 216,406 |
| Queensland | 3,759,098 | 199,374 | 6,566,726 |
| South Australia | 1,736,266 | 83,916 | 1,995,313 |
| Tasmania | 598,116 | 22,449 | 619,589 |
| Victoria | 5,916,835 | 234,852 | 8,495,137 |
| Western Australia | 2,408,043 | 104,757 | 3,525,705 |
| 20,468,530 | 1,227,247 | 33,812,245 | |
| As of 18 March 2022 14:00 AEST[14] | |||
Australian Capital Territory

On 22 February 2021, the first Canberran received a COVID-19 vaccination. She was a 22-year-old registered nurse, and a member of a COVID-19 testing team.[33]
In the ACT, by 11 June 2021, one mass vaccination clinic centre in Garran, one vaccination clinic in Calvary Public Hospital and some selected GP clinics were delivering vaccinations.[34]
60% of the population of the ACT had received their first dose as of 24 August 2021.[9] On 11 September 2021, the ACT became the first Australian state or territory to have 50% of the eligible adult population over 16-years-old fully vaccinated.[35] On 30 September 2021, the ACT became the first state or territory to reach 90% of first doses administered to the 16-years or older eligible population.[36]
New South Wales
On 8 April 2021, the Australian Technical Advisory Group on Immunisation (ATAGI) recommended that the Pfizer vaccine (Comirnaty) be preferred over the AstraZeneca vaccine in people under the age of 50.[37] This led to the NSW government to temporarily suspend inoculation with the AstraZeneca vaccine in the state for one day.[38]
On 10 May 2021, a mass vaccination hub opened at Sydney Olympic Park. The same day, registrations began for NSW residents aged 40 to 49 to receive the Pfizer vaccine.[39] On 9 August, Qudos Bank Arena at Sydney Olympic Park was opened as a Pfizer vaccination hub for Higher School Certificate (HSC) students. The hub was fully booked with almost 3,000 appointments on its first day.[40] The Qudos Arena vaccination hub closed on 7 November 2021, after delivering more than 360,000 doses.[41]
From 16 June 2021, NSW residents who were aged over 50 could get an AstraZeneca vaccination from selected pharmacies. The NSW health department approved 1,250 pharmacies to administer the vaccine under strict regulations.[42]
On 12 July, the state government opened up the AstraZeneca vaccine to over-40s, with vaccination hubs opening in the Fairfield, Canterbury-Bankstown and Liverpool regions, amid a growing outbreak in those areas of the Delta variant of COVID-19.[43][44]
On 24 July, ATAGI released a statement in response to the NSW Delta outbreak, which stated that all individuals aged 18 years and over in greater Sydney, including adults under 60 years of age, should strongly consider getting vaccinated with any available vaccine, including the AstraZeneca COVID-19 Vaccine. This was based on an increased risk of COVID-19 infection and ongoing constraints in supplies of the Pfizer vaccine.[45]
On 24 August, NSW reached the milestone of 60% first doses administered to the eligible population.[46] On 2 September NSW became the first state to reach the 70% level of first doses given to their eligible population.[47]
By 5 September 2021, 40% of the NSW population was fully vaccinated.[48] On 15 September 2021, NSW became the first state in the country to have 80% of its population having at least one vaccine dose.[49] By 17 September 2021, 50% of the 16 years, or older, population of NSW had received two vaccine doses.[50]
On 26 September 2021, 60% of eligible residents became fully (double dose) COVID-19 vaccinated. 85% had a single vaccine dose.[51]
On 27 September 2021, the three-stage roadmap to come out of lockdown, and freedoms for vaccinated versus unvaccinated people, was announced by then Premier Gladys Berejiklian. All three stages depend upon reaching the double-dose vaccination rates of 70, 80 and 90%.[52]
On 7 October 2021, 70% of eligible residents who were aged 16 and over became fully vaccinated against COVID-19.[53] 2 days later, the same demographic reached 90% having had at least one dose.[54] On 11 October 2021, NSW moved to Phase Two - Vaccination Transition Phase as the state achieved 70% full vaccination of the eligible population the previous week. Premier (as of 5 October) Dominic Perrottet announced "Freedom-day" for NSW as the state came out of lockdown and restrictions were eased, but only for fully vaccinated people, across the state. Some restrictions remained in place until the 80 and 90% levels of vaccination were reached.[55]
On 16 October 2021, 80% of eligible state residents became fully vaccinated against COVID-19. NSW was the first state or territory to achieve 80% full vaccination. The single dose vaccination rate in NSW was 91.9% on 15 October.[56]
On 8 November 2021, a new COVID-19 vaccination clinic opened at The Granville Centre after the closing of the Qudos Bank Arena clinic on 7 September. The Granville clinic will open between 8 am and 4 pm, seven days a week for first, second or booster doses.[41]

Northern Territory
On 22 February 2021, the first COVID-19 vaccinations (phase 1A) in the Northern Territory (NT) were administered to "at-risk frontline workers" using the Pfizer–BioNTech COVID-19 vaccine.[57] As of 13 June 2021, 25 general practices and 3 respiratory clinics were delivering vaccinations across the territory.[58]
Queensland
By 13 June 2021, the Queensland Health Department was delivering vaccines under phase 1a, 1b & 2a (people aged over 40) in the state. Age 40+ vaccination centres including hospitals, event centres and GP clinics. Eligible residents can register their interest in vaccinations online or make an appointment at the nearest centre. Queensland Premier Annastacia Palaszczuk said that preparation is underway to establish a mass vaccination centre by the end of 2021.[59]
The state's first mass vaccination hub opened at the Brisbane Convention & Exhibition Centre on 11 August.[60][61]
South Australia
On 5 May 2021 the first Oxford-AstraZeneca vaccination was administered at Murray Bridge, South Australia. The recipient was a doctor in regional SA.[62]
On 19 March 2021, South Australia faced a setback due to a misdirected shipment of vaccines. The Pfizer vaccine was supposed to go to Adelaide but was wrongly delivered to Perth, Western Australia. Premier of South Australia Steven Marshall denied knowledge of any delivery and said it was a federal government responsibility to deliver the vaccine. Federal officials confirmed the misdirected delivery.[63]
On 30 April 2021, South Australia's first COVID-19 mass vaccination hub opened at Adelaide Showground.[64]
Tasmania
By 11 June 2021, the Tasmanian Health Department was conducting vaccinations according to the national vaccine roll-out plan in a phased manner. Those eligible can book an appointment online or over the hotline number. Hospitals, clinics, community health centres and GP clinics (for ages 50+) are participating in the vaccination program.[65]
On 16 September 2021, Tasmania became the second state, after the Australian Capital Territory,[35] to achieve 50% full vaccination of the 16 years and older population.[66]
Victoria

On 21 April 2021, Victoria's first three mass vaccination centres were opened at the Melbourne Convention & Exhibition Centre, the Royal Exhibition Building in Carlton and in Geelong. Those eligible for vaccination can make an appointment over the phone or walk in at any centre.[67] One more mass vaccination centre for central Victoria was due to be opened in Bendigo by the end of May.[68]
On 28 May, the state expanded its vaccine rollout to adults aged 40 and over, ahead of the federal rollout timeline.[69][70]
On 9 August, the AstraZeneca vaccine became available to adults aged 18–39 at state-run vaccination hubs. The Pfizer vaccine was also made available for immuno-compromised children between the age of 12 and 15 at the same mass vaccination centres.[71][72] On the same day, Australia's first drive-through mass vaccination hub opened at the site of a former Bunnings store in Melton. It initially offered Pfizer doses for those aged 40 and above, with AstraZeneca doses expected a week later.[73]
Anyone who is an adult (16 years old and over) became eligible to get the Pfizer vaccine from 25 August. As per the announcement made by the state government, 16 and 17-year-olds are only allowed the Pfizer vaccine, while 18 to 59-year-olds can choose between the AstraZeneca or Pfizer vaccines.[74]
On 17 September 2021, Victoria reached the milestone of 70% partially vaccination of the 16+ eligible population.[75]
Western Australia

As of 10 June 2021, people aged 30 years and over, as well as the below groups aged 16 years and over, are eligible for COVID-19 vaccination.[76][77]
- People with a specified underlying medical condition.
- Carers of people with specified underlying medical conditions and disabilities.
- Essential outbound travellers with a travel exemption.
- Critical and high-risk workers.
- Health care, aged care, disability care workers and volunteers.
- Quarantine and Border workers including household contacts.
- Aboriginal and Torres Strait Islander people.
- Pregnant women are aged 16 and over.
Metropolitan community clinics, GP respiratory clinics, GP clinics, Aboriginal Medical Services and regional community clinics are participating in the vaccination rollout.[78]
Children (that is, aged below 18 years) are offered Pfizer-BioNTech, adults aged between 18 and 59 years are offered Pfizer-BioNTech and AstraZeneca, and adults aged 60 and over are offered AstraZeneca.[77][78]
On 16 August, Western Australia expanded eligibility for the Pfizer vaccine to anyone aged 16–29.[79]
Western Australians aged 60 and above became eligible for the Pfizer vaccine from 20 September.[80][81]
Vaccine approval
The four vaccines currently approved for administration in Australia are classified as being "provisionally approved", meaning that they have been deemed both safe and effective based on clinical and scientific data and are in the process of non-expiring registration. The authorisation means the vaccine will become part of the Australian Therapeutic Goods Register and will be up for review again in two years based on additional clinical data.[82]
Pfizer–BioNTech vaccine
On 25 January 2021, the TGA provisionally approved the two-dose Pfizer–BioNTech COVID-19 vaccine, named COMIRNATY, for use within Australia. The provisional approval only recommends the vaccine for patients over the age of 16, pending ongoing submission of clinical data from the vaccine sponsors (the manufacturers, Pfizer and BioNTech).[83] Additionally, every batch of vaccines have their composition and documentation verified by TGA laboratories before being distributed to medical providers.[84]
The Department of Health and Aged Care planned the administration of COVID-19 vaccinations in five phases, organised by the risk of exposure. Border, quarantine, and front-line health and aged care workers were vaccinated first, followed by over 70 year-olds, other health care workers, and essential emergency service members. Following the provisional approval of COMIRNATY, Prime Minister Scott Morrison said that it was planned for the first group to begin vaccinations by February 2021, six weeks earlier than originally planned.[85]
The first public COVID-19 vaccination in Australia actually took place on 21 February 2021 with the Pfizer–BioNTech vaccine at Castle Hill in Sydney. An 84-year-old aged care resident was the first Australian to receive the vaccine. To show confidence in the national immunisation vaccine rollout, Prime Minister Morrison and Chief Medical Officer Professor Paul Kelly also received vaccinations.[86]
On 23 February 2021, Australia's second shipment of the Pfizer vaccine arrived at Sydney airport. Health Minister Hunt confirmed the arrival of 166,000 doses, and 120,000 more doses expected to arrive in the following week.[87]
On 9 April 2021, Prime Minister Morrison announced that Australia had secured another 20 million doses of Pfizer vaccine on top of 20 million already on order, meaning 40 million doses should be available to Australians in 2021. This was amid concerns about the AstraZeneca vaccine, in rare cases, causing blood clots; see section Oxford–AstraZeneca vaccine below. The additional doses of Pfizer were expected to arrive in Australia in the last quarter of 2021.[88][89]
On 23 July 2021, the TGA approved the Pfizer COVID-19 vaccine for teenagers between 12 and 15 years old.[90]
On 5 December 2021, the TGA provisionally approved the Pfizer COVID-19 vaccine access for five to 11-year-olds.[91][92]Oxford–AstraZeneca vaccine
_I_(cropped).jpg.webp)
On 16 February 2021, the Oxford–AstraZeneca vaccine was approved by the TGA for use in Australia. The administration of this vaccine was scheduled to start in March.[93] Two weeks later, on 28 February, the first shipment of the vaccine, around 300,000 doses, arrived at Sydney for rollout from 8 March.[94] On 5 March 2021, Italy stopped the export of AstraZeneca vaccine to Australia due to their slower rollout of that vaccine in the EU.[95] On 23 March, TGA approved the first batch of locally manufactured AstraZeneca vaccine by CSL-Seqirus in Melbourne, and 832,200 doses were ready for rollout in the following weeks.[96]
On 17 June 2021, Federal Health minister Greg Hunt announced a rise in the age limit for administration of the AstraZeneca vaccine. After new advice from the Australian Technical Advisory Group on Immunisation (ATAGI), the vaccine was no longer recommended for people aged under 60 years. This advice came after new cases of blood clotting, thrombosis with thrombocytopenia syndrome (TTS), in those under 60 after AstraZeneca vaccinations.[89]
On 23 June 2021, the Federal government released vaccine allocation projections and forecast that the Oxford-AstraZeneca vaccine would be in "little need" past October 2021 when all Australians over 60 years were expected to be fully vaccinated.[97]
On 9 February 2022 within Australia the Oxford-AstraZeneca vaccine was approved by the TGA (still pending ATAGI approval) as booster vaccines for individuals – joining Pfizer and Moderna booster vaccines for individuals approved months ago.[98]Moderna vaccine
On 24 June 2021, the Moderna COVID-19 vaccine, Elasomeran, was issued a provisional determination by the TGA making it eligible to apply for provisional registration in Australia. It is targeted for use in individuals aged 18 years of age and older pending approval.[99] The Moderna vaccine was approved in Australia for 18 years or older by the TGA on 9 August 2021. It was also approved for adolescents aged between 12 and 17 on 4 September 2021.[7][100]
Janssen (J&J) vaccine
On 25 June 2021, provisional approval was given by the TGA to the Janssen COVID-19 vaccine, the third vaccine for potential use in Australia. Strict conditions were imposed on Janssen, which includes further investigation documents related to the efficacy, long term effects and safety concerns that must be provided regularly to TGA.[8] As of 3 August 2021 following the release of the 'Op COVID SHIELD National COVID Vaccine Campaign Plan', it is not included in the vaccination program.[101]
Novavax vaccine
On 20 January 2021, the Novavax vaccine was issued a provisional determination by the TGA making it eligible to apply for provisional registration in the Australian Register of Therapeutic Goods (ARTG).[102] As of 14 June 2021, Novavax had entered the final stage of trials.[103] As of 8 July 2021, the Novavax COVID-19 vaccine was under evaluation by the TGA for use in Australia.[104] As of 23 January 2022, the TGA gave provisional approval and as of 24 January, ATAGI has approved it's rollout to start when shipments arrive in late February.[105] Currently Novavax is available free of charge to self described Australian citizens with or without Medicare. Walk in clinics are available in capital cities.
ATAGI recommendation on vaccine use
After the TGA approves vaccines, the Australian Technical Advisory Group on Immunisation (ATAGI) provides recommendations and clinical guidance on vaccine use. The recommendation as of 29 July 2021 is that the AstraZeneca COVID-19 vaccine is the preferred vaccine for people aged 60 years and older. The Pfizer COVID-19 vaccine is the preferred COVID-19 vaccine in people aged under 60 years of age; and is additionally recommended in people with a history of cerebral venous sinus thrombosis (CVST), heparin-induced thrombocytopenia (HIT), deep vein thrombosis or antiphospholipid syndrome with thrombosis. These recommendations are based on the risk of TTS appearing to be higher in younger adults than in older adults, and younger adults having a lower likelihood of having severe outcomes from COVID-19 compared to older adults and theoretical concerns that a history of the rare conditions listed above may increase the risk of TTS.[106] The AstraZeneca vaccine is also recommended to most people aged 18 years after consultation with their GP who are resident in declared hotspot areas.[107]
In June 2021, the Federal government projected that the Oxford-AstraZeneca vaccine would see "little need" after October 2021 when all over 60 year-old Australians were expected to be immunised.[108]
International Vaccines recognition
On 1 October 2021, Prime Minister Scott Morrison announced that the Therapeutic Goods Administration has considered two international vaccines for future international travel as equal to vaccines approved to use in Australia to skip strict hotel quarantine. The first vaccine is Indian made Astrazeneca vaccine under the brand name "COVISHIELD", and the second is CoronaVac from China.[109]
On 1 November 2021, the TGA recognised two more vaccines: Covaxin and the Sinopharm BIBP vaccine (BBIBP-CorV).[110]
On 17 January 2022, the TGA recognised the Sputnik V vaccine.[111]
| Vaccine name | Country of origin | Manufacturer | Status |
|---|---|---|---|
| Covishield | Astrazeneca/Serum Institute of India | ||
| Covaxin | Bharat Biotech | ||
| CoronaVac | Sinovac Biotech | ||
| BBIBP-CorV | Sinopharm | ||
| Sputnik V | Gamaleya Research Institute of Epidemiology and Microbiology | ||
| Janssen | Janssen Pharmaceuticals |
Vaccine supply & issues
The Australian government entered into agreements with Pfizer/BioNTech, University of Oxford/AstraZeneca, Novavax, the University of Queensland and COVAX for the supply of vaccines.[112] The University of Queensland vaccine was abandoned in December 2020 after trials revealed that, while it was safe, it triggered false positives on HIV tests.[113][114] The Therapeutic Goods Administration (TGA) provisionally approved the Pfizer vaccine in January 2021.[115] The Australian government ordered 10 million doses, with the first 80,000 to be delivered in February 2021, but production problems and the imposition of export controls by the European Union (EU) onto deliveries to countries outside Europe made meeting the delivery schedule problematic.[116]
Delivery issues also affected deliveries of the Oxford–AstraZeneca COVID-19 vaccine, which was provisionally approved by the TGA in February,[117] and received final approval in March.[118] Orders were reduced from 3.8 million to 1.2 million doses of this vaccine, which was manufactured in Belgium,[116][119] and arrival was pushed back to March 2021.[120] CSL Limited began manufacturing 50 million doses of the AstraZeneca vaccine in Melbourne in November 2020. Deliveries were expected to commence in March.[121] The AstraZeneca vaccine could be stored at normal refrigeration temperatures of 2 to 8 °C (36 to 46 °F), whereas the Pfizer vaccine required storage at −70 °C (−94 °F).[120] However, concerns were raised about the efficacy of the AstraZeneca vaccine.[122][123] The Immunology & Cell Biology called for a pause in its rollout, as the efficacy of the vaccine reported by trials was insufficient to achieve the desired herd immunity effect.[124] CSL management declined an invitation to appear before an Australian Senate inquiry.[125]
Although the Prime Minister of Australia, Scott Morrison, said that Australia would be "at the front of the queue",[126] and the Minister for Health and Aged Care, Greg Hunt, claimed that Australia would be among the first countries to receive COVID-19 vaccines, 61 other countries had already commenced vaccinating their citizens by the end of January 2021, while the Australian vaccination rollout was not scheduled to commence for another month.[125]
On 15 February 2021, 142,000 doses of the Pfizer–BioNTech COVID-19 vaccine arrived in Australia. The first doses were due to be administered on 22 February.[127] The world-wide distribution of the vaccine has been described as "the largest logistics effort in the world since World War" by Dr Roberto Perez-Franco of the Deakin University's Centre for Supply Chain and Logistics.[128] This was followed, on 28 February, by 300,000 doses of the Oxford–AstraZeneca vaccine, which arrived at Sydney airport. It was planned that most Australians would be vaccinated with this vaccine, the majority manufactured in Australia by CSL Limited.[129] On 5 March, Italy and the European Union blocked a shipment of 250,000 doses of the Oxford−AstraZeneca vaccine from Italy to Australia, citing low COVID-19 case numbers in Australia and the limited availability of vaccines in the EU.[130]
Local manufacturing began in November 2020.[131][132] On 16 February, the first vials of COVID-19 vaccine produced in Australia came off the production line at the CSL Behring plant in Broadmeadows, Melbourne.[133] This is the active raw vaccine material. The vaccine vials are filled and packaged into doses by Seqirus, a CSL subsidiary in Parkville, Melbourne.[134] Production of the AstraZeneca vaccine in Australia received its final approval from the TGA on 21 March.[118] Some front line health care workers were reported to have preferred the Pfizer vaccine over the AstraZeneca one.[135]
The Australian government had also signed a deal with Novavax for 51 million doses of its vaccine, with supply originally slated for "mid-2021".[136] As of April 2021, it had yet to be approved by the TGA. It is not manufactured in Australia, so like the other imported vaccines, its availability was uncertain. In trials it was reported to be 95.6 per cent effective against COVID-19, and an 86.3 per cent effective against the variant identified in the UK.[136] Australia's first human trials of a candidate COVID-19 vaccine was Novavax's NVX-CoV2373 which began in Melbourne by 26 May 2020.[137]
In a February 2021 pre-budget submission, the Australian Academy of Science renewed its call for the government to develop the capability to produce mRNA vaccine technology in Australia. The ability to mass-produce such vaccines onshore would insulate Australia against supply shocks, and cater for future pandemics and potential biosecurity situations. The mRNA coronavirus vaccines made by Moderna and Pfizer showed strong results in clinical trials and are expected to be easier to reconfigure to cater for new virus variants than more conventional vaccines.[138][139]
The US Moderna company entered an agreement with the Australian Federal government, announced on 13 May 2021, to provide 25 million doses of its COVID-19 vaccine 'mRNA-1273', subject to TGA approval.[140]| Vaccine name | Status | Quantity | Vaccine approved | Began administering | Age group | Booster dose approval | Ref |
|---|---|---|---|---|---|---|---|
| Pfizer–BioNTech | 125 million | Aged 5+[141] | [142][143][144][145] | ||||
| Oxford–AstraZeneca | 53.8 million | Aged 18+ | [147][148] | ||||
| Moderna | 25 million | Aged 6 months+ (eff. 5 September)[149][150][151] | [152][7][153][154][155][156] | ||||
| Novavax | 51 million | Aged 12+[160] | [162][163] |
Vaccination timeline criticisms
On 11 March 2021, the Australian Medical Association (AMA) attested that it was implausible that the government's target of offering vaccination to every Australian by October 2021 would be achieved, and suggested that mid-December 2021 would be a more realistic date. The government had aimed to administer 60,000 doses by the end of February but administered only 31,000 doses.[164] The vaccination program was also 83.25% behind its target figure by the end of March: 4 million doses were targeted by the Health Department before the rollout, but only 670,000 had been delivered.[165]
The vaccination rollout had a further setback when pharmacists postponed joining the vaccination program until June.[166] The federal government said that the European Union (EU) blocking the shipment of more than 3 million doses of vaccine to Australia was a major reason for the delayed vaccine rollout, although the EU only officially confirmed blocking the export of 250,000 doses in early March.[167][168]
Medical advice discouraging the use of the AstraZeneca vaccine on people under the age of 50 due to incidents of vaccine-related blood clotting was a further major setback in the vaccination rollout, given the AstraZeneca vaccine was originally slated as the cornerstone of the entire program. Prime Minister Scott Morrison stated at the time that a definitive timeline for vaccine rollout could no longer be provided, and there is a need to re-evaluate and recalibrate the program.[169]
On 11 April 2021, Prime Minister Morrison conceded the earlier target to vaccinate all Australians by the end of 2021 was difficult to achieve, also saying there was no set target for the vaccination timeline due to the many uncertainties involved.[12] Morrison suggested two meetings of the National Cabinet be held per week until all issues delaying the vaccine rollout were fixed.[170]
More than two million COVID-19 vaccinations had been administered by 28 April 2021, but this was three million short of original plans.[11] The federal government was criticised by some for declining an invitation to meet with Pfizer executives in 2020, at a time other countries were starting to place orders.[171]
As of 16 August 2021, more than 10 million Australians had received their first dose of COVID-19 vaccines across the nation.[172]
Vaccine candidates in clinical trials
As of 2 October 2022, there were nine vaccine candidates registered to conduct in clinical trials in Australia, but not all had begun enrollment of trial participants.[173]
| Vaccine | Country of origin | Type (technology) | Progress | Ref |
|---|---|---|---|---|
| RBD SARS-CoV-2 HBsAg VLP SpyBiotech |
United Kingdom | Virus-like particle | Phase I–II (280) Randomized, placebo-controlled, multi-center. Aug 2020 – 2021, Australia |
[174] |
| COVAX-19 Vaxine Pty Ltd |
Australia | Subunit (recombinant protein) | Phase III (16,876) Randomized, Two-armed, Double-blind, Placebo controlled Aug – Sep 2021, Iran |
[175] |
| INNA-051
Ena Respiratory |
Australia | Viral vector | Phase II (423) Randomized, double-blind, placebo-controlled. Mar – Dec 2022, Australia |
[176] |
| COVIGEN University of Sydney |
Australia | DNA | Phase I (150) Double-blind, dose-ranging, randomised, placebo-controlled. Feb 2021 – Jun 2022, Australia, Thailand |
[177] |
| bacTRL-Spike Symvivo |
Canada | DNA | Phase I (24) Randomized, observer-blind, placebo-controlled. Nov 2020 – Feb 2022, Australia |
[178] |
| SC-Ad6-1
Tetherex Pharmaceuticals |
United States | Viral vector | Phase I (40) First-In-Human, Open-label, Single Ascending Dose and Multidose. Jun – Dec 2021, Australia |
[179] |
| IVX-411
Icosavax, Seqirus Inc. |
United States | Virus-like particle | Phase I–II (168) Randomized, observer-blinded, placebo-controlled. Jun 2021–2022, Australia |
[180] |
| COVID-19-EDV EnGeneIC |
Australia | Viral vector | Phase I (18) Open label, non-randomised, dose escalation. Aug 2021–Jan 2022, Australia |
[181] |
| Unnamed Indian Immunologicals, Griffith University |
Australia, India | Attenuated | Preclinical |
[182] |
Vaccine passport
In June 2021, the federal government revealed that they planned to introduce a Digital Vaccine Passport in the future as proof of vaccination. All vaccinated Australians would be able to access their digital vaccine certificates through the Express Plus Medicare app or myGov account. Governments that have stated their intention to have a similar system are Canada, the European Union, and the UK. Fully vaccinated persons can also add their digital certificate in their Apple Wallet or Google Pay.[183][184]
In September 2021, South Australia began trialling a Digital Passenger Declaration (DPD) that could replace the physical Incoming Passenger Card and the digital COVID-19 Australian Travel Declaration form. This declaration would be completed by all incoming travellers and would take the form of a mobile or web app. The DPD would contain a digital vaccination certificate, and could also be used to track home quarantine and assist with contact tracing.[185]
In October 2021, National Cabinet announced that the Australian government would create an International COVID-19 Vaccine Certificate for outgoing travellers which would follow standards specified by the International Civil Aviation Organization.[186] On 19 October 2021, International COVID-19 Vaccine Certificate was made available for Australian passport holders and visa holders with a QR code and it can be downloaded from the MyGov website or medicare express app.[187] The QR code on International COVID-19 Vaccine Certificate can be scanned and verified by using VDS-NC app which is available now on App store and google play store.[188][189]

Vaccination and Australia's reopening
On 30 July 2021, the federal government released a revised four-phase plan to transition Australia's National COVID-19 Response from its current pre-vaccination settings, focussing on continued suppression of community transmission, to post-vaccination settings focussed on prevention of serious illness, hospitalisation and fatality, and the public health management of other infectious diseases. The phases transitions are triggered in a jurisdiction when the average vaccination rates across the nation have reached the threshold and that rate is achieved in a jurisdiction expressed as a percentage of the eligible population (16+), based on the scientific modelling conducted for the COVID-19 Risk Analysis and Response Task Force. As of 6 November 2021, Australia is in phase-Three, which is "Vaccination Consolidation Phase".[190][191]
In a statement by the Prime Minister on 30 July 2021, it was announced that the federal governments and all states and territories had agreed in-principle to the updated plan.[192]
There has been no date set for each phase. The percentage fully vaccinated eligible population to transition into the second phase, Phase B, is 70%, and 80% into the third phase, Phase C.[193][190] No target was decided for Phase D instead being sporadically put into effect from 21 February to 6 July when unvaccinated travellers were freely allowed to enter Australia.[194][195]
On 16 November 2021, the percentage of the eligible adult population aged 16 and older fully vaccinated reached 83.9%.[32]
On 23 March 2022, the percentage of the eligible adult population aged 16 and older fully vaccinated reached 95.0%[15]
| Measures may include | Estimated start date | Target percentage of fully vaccinated eligible adult population (16+) [lower-alpha 5] | Status |
|---|---|---|---|
| Phase One - Vaccinate, prepare and pilot | |||
|
1 July 2021 – 19 October 2021 | Phase Completed | |
| Phase Two - Vaccination Transition Phase | |||
|
20 Oct 2021 – 5 Nov 2021 | Phase Completed | |
| Phase Three - Vaccination Consolidation Phase | |||
|
6 Nov 2021 – 6 July 2022 | Phase Completed | |
| Phase Four - Post-Vaccination Phase (Back to Normal) | |||
|
6 July 2022 – present | Phase Completed | |
Operation COVID Shield
On 3 August 2021, the Australian Government publicly released the 'Operation COVID SHIELD National COVID Vaccine Campaign Plan'[101] and the Doherty Institute Modelling Report to advise on the National Plan to transition Australia's National COVID Response'.[196]
Adverse Events Following Immunization (AEFI)
Approved COVID-19 vaccinations are considered safe. There are strict protections in place to help ensure the safety of all COVID-19 vaccines, including clinical trials to meet the benchmark of safety nationally and internationally. COVID-19 vaccines can cause mild, short term side effects, such as a low-grade fever or pain or redness at the injection site much like other vaccines and injected medications. Most reactions to vaccines are mild and go away within a few days on their own. More serious or long-lasting side effects to vaccines are possible but extremely rare. Vaccines are continually monitored for as long as they are in use, to detect rare adverse events and implement approaches to limit their occurrence.[197]
Possible side effects due to vaccination
- Common side effects can include headache, muscle pain, fever, chills, muscle pain, lethargy, injection site reactions and fatigue
- Very Rare side effects can include Thrombosis with thrombocytopenia syndrome (TTS), Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the membrane around the heart)
| AEFI reporting rate per 1000 doses | 2.2 |
|---|---|
| Total AEFI reports received | 85,714 |
| Total Doses administered | 39,106,606 |
| Total reports for Vaxzevria (AstraZeneca) vaccine | 41,598 |
| Total reports for Comirnaty (Pfizer) | 41,762 |
| Total reports for Spikevax (Moderna) | 2,013 |
| Total reports for TTS | 166 |
| Total death reports due to TTS | 8 |
| Total reports for Guillain-Barre Syndrome (GBS) | 156 suspected |
| Total reports for myocarditis | 730 suspected cases [lower-alpha 6] |
| Total reports for pericarditis | 1,544 suspected cases [lower-alpha 6] |
| Total reports for Immune thrombocytopenia (ITP) | 93 suspected cases[lower-alpha 6] |
| Total death reports due to ITP | 1 |
| Total death reports | 686 |
Reported events
One of the earliest reported serious adverse events in Australia was a 44-year-old man admitted to Melbourne's Box Hill Hospital on 2 April 2021 when he developed serious thrombosis and thrombocytopenia syndrome (TTS) [low platelet count] after receiving the AstraZeneca vaccine on 22 March. Similar cases had been reported overseas among those who received the AstraZeneca vaccine. The event prompted the TGA to warn anyone who experienced persistent headaches or other worrying symptoms 4 to 20 days after receiving the vaccine to seek medical advice.[198][199]
In April, a 48-year-old woman died in John Hunter Hospital on 15 April after developing TTS four days after vaccination. This was the first death in Australia likely linked to COVID-19 vaccination. It was also confirmed that the woman had diabetes and had other underlying medical conditions.[200] Since then further cases of serious adverse events have been occasionally reported in the media,[201][202][203][204] and two more deaths following COVID-19 vaccination identified in June. A 52-year-old woman whom died on 10 June due to a blood clot in her brain (cerebral venous sinus thrombosis). The TGA stated it was "likely" the death was linked to a rare vaccination side-effect. This was the second death in Australia linked to the Oxford-AstraZeneca vaccine.[205] On 17 June, this partly led to a federal government decision to only recommend the AstraZeneca vaccine for those over 60 years-of-age on advice from ATAGI.[206] In late June, a 61-year-old woman died in Royal Perth Hospital from immune thrombocytopenic purpura (ITP), which the TGA stated was likely linked to her AstraZeneca vaccination.[207][208]
In July 2021, three deaths linked by the TGA following AstraZeneca vaccination were reported. A 72-year-old woman vaccinated on 24 June was admitted to Royal Adelaide Hospital on 5 July and died six days later.[209] Another two deaths were later identified of people in their 40s.[210]
Ongoing updates on reviews by ATAGI into confirmed and suspected adverse events are publicly available. As of 4 August 2021 update, ATAGI reinforces that the benefits of vaccination with COVID-19 Vaccine AstraZeneca strongly outweigh the risks of adverse effects in all Australians aged 60 years or older.[211]
Changes to AstraZeneca vaccine recommendations

After findings and advice on the AstraZeneca vaccine from the UK and EU were released following several months of data on their vaccine rollout, the Australian Technical Advisory Group on Immunisation (ATAGI) and TGA met on 8 April 2021 to review and advise the government as a part of the periodic review process. Australia's Chief Medical Officer, Paul Kelly, reassured the safety of the vaccine and noted it was being continually reviewed, and that other vaccine options like Pfizer Comirnaty vaccine, and potentially later, Novavax, existed for the nation.[212]
Everyone aged over 50-years were encouraged to get the AstraZeneca vaccine,[213] until 17 June when ATAGI's recommendation was revised to people over 60.[206] Internationally there is no consensus on the age limit.[214][215] The Department of Health information sheet on the AstraZeneca vaccine published 30 July, states "in outbreak settings, adults under 60 years of age should strongly consider AstraZeneca Vaccine if they are unable to access Comirnaty (Pfizer vaccine)".[216]
See also
Notes
- ↑ Front-line workers is limited to staff at border and quarantine facilities, health care staff in emergency and COVID-19 wards in hospitals, and other direct-contact workers.
- ↑ Both doses
- ↑ Figure shows eligible got at least one dose of the approved vaccine
- ↑ Recommended for over 60s, and over 18s in greater Sydney.[45]
- ↑ Phase progression depends upon the achievement of the threshold of certain vaccination percentage, scientific modelling of vaccination threshold is completed at Doherty Institute
- 1 2 3 Under TGA scrutiny
References
- ↑ Australia COVID- 19 Vaccine Tracker: States Map. By George Karabassis. Press the % button and Fully vaccinated dose button from drop down to create the shown map.
- ↑ "COVID-19 and the Australian Government's Response". Budget 2021–21. Archived from the original on 6 October 2020. Retrieved 20 February 2021.
- 1 2 "COVID-19 vaccines". Department of Health. 13 August 2021. Retrieved 30 August 2021. (NB: The data on this site changes daily)
- ↑ "TGA provisionally approves Pfizer/BioNTech COVID-19 vaccine for use in Australia". Department of Health. 25 January 2021. Archived from the original on 26 January 2021. Retrieved 26 January 2021.
- ↑ "TGA provisionally approves AstraZeneca COVID-19 vaccine for use in Australia". Department of Health. 16 February 2021. Archived from the original on 16 February 2021. Retrieved 17 February 2021.
- ↑ Hitch, Georgia (7 January 2021). "When will I get the coronavirus vaccine? Who gets the vaccine first?". ABC News. Retrieved 26 January 2021.
- 1 2 3 Marsh, Stuart (10 August 2021). "Moderna COVID-19 vaccine provisionally approved by the TGA for use in Australia". 9 News Australia. Retrieved 10 August 2021.
- 1 2 3 "TGA grants third provisional approval to COVID-19 vaccine: Janssen". tga.gov.au. 25 June 2021. Retrieved 6 July 2021.
- 1 2 3 4 5 6 7 8 9 10 "Australia Covid vaccine tracker". COVIDLIVE. 13 March 2021. Archived from the original on 18 February 2021. Retrieved 13 August 2021.(NB: The data on this site changes daily.)
- ↑ "Tracking Australia's COVID vaccine rollout numbers". Australian Broadcasting Corporation. 1 September 2021. Retrieved 1 September 2021. (NB: The data on this site changes daily)
- 1 2 "Australia administers 2 million COVID-19 vaccine doses, well short of initial targets". ABC News. 1 May 2021. Retrieved 7 May 2021.
- 1 2 "Vaccine targets dumped: Morrison concedes all Australians may not be vaccinated by the end of the year". The Sydney Morning Herald. 11 April 2021. Retrieved 14 April 2021.
- ↑ "Australia still lags many other countries on vaccine rollout – but it's catching up fast". The Guardian. 11 October 2021. Retrieved 12 October 2021.
- 1 2 "Australia Covid vaccine tracker by source". COVIDLIVE. 13 September 2021. Archived from the original on 11 June 2021. Retrieved 13 September 2021.(NB: The data on this site changes daily.)
- 1 2 "Vaccination numbers and statistics | Australian Government Department of Health". 23 March 2022. Archived from the original on 23 March 2022. Retrieved 4 August 2022.
- ↑ "How COVID-19 vaccines will be distributed". Department of Health. 18 February 2021. Archived from the original on 27 January 2021. Retrieved 21 February 2021.
- 1 2 "COVID-19 vaccine national rollout phases". Australian Government Department of Health. 22 April 2021. Retrieved 25 April 2021.
- ↑ "COVID-19 vaccination – Australia's COVID-19 vaccine national roll-out strategy". Australian Government Department of Health. 17 February 2021. Retrieved 12 March 2021.
- ↑ "Australian PM is vaccinated as rollout begins". BBC News. 21 February 2021. Archived from the original on 21 February 2021. Retrieved 21 February 2021.
- ↑ "MPhase 1B rollout underway as 'workhorse' AstraZeneca vaccine approved to be locally produced". 9NEWS. 22 March 2021. Retrieved 22 March 2021.
- ↑ "COVID-19 vaccines to be made available for 16-39-year-olds by end of month". 9NEWS. 19 August 2021. Retrieved 19 August 2021.
- ↑ "Melbourne Press Conference with Minister Hunt and Professor John Skerritt, on 5 December 2021, on the TGA provisionally approving Pfizer COVID-19 vaccine for 5 to 11-year-olds". Department of Health. 5 December 2021. Retrieved 5 December 2021.
- ↑ "Australia vaccinating children against COVID-19 from early next year". Australian Government Department of Health. 10 December 2021. Retrieved 22 December 2021.
- ↑ "National, state and territory population". Australian Bureau of Statistics. 17 June 2021. Retrieved 19 June 2021.
- 1 2 3 4 5 6 7 8 9 "COVID-19 vaccine weekly safety report - 02-12-2021". Therapeutic Goods Administration. 2 December 2021. Archived from the original on 2 December 2021. Retrieved 4 December 2021.(NB: The data on this site changes weekly.)
- ↑ "Australia begins rollout of Pfizer vaccine". 9news.com. Retrieved 21 February 2021.
- ↑ "Queensland will begin the COVID-19 vaccination program tomorrow". www.youtube.com. Retrieved 21 February 2021.
- ↑ "COVID vaccine arrives in South Australia". www.youtube.com. Retrieved 21 February 2021.
- ↑ "Pfizer vaccine to arrive in Tasmania on Monday with 1000 doses to be rolled out to priority workers from Tuesday". www.abc.net.au. 18 February 2021. Retrieved 21 February 2021.
- ↑ "Pfizer vaccine arrives in Victoria". youtube.com. Retrieved 21 February 2021.
- ↑ "Pfizer vaccine arrives in Victoria". 9news.com. Retrieved 21 February 2021.
- 1 2 Macali, Anthony (4 December 2021). "Coronavirus Cases in Australia". covidlive.com.au. Retrieved 4 December 2021.
- ↑ Vidot, Anna (23 February 2021). "Meet the nurse who received Canberra's first COVID vaccination". ABC Radio Canberra. No. Drive. Australian Broadcasting Corporation. Retrieved 25 February 2021.
- ↑ "Booking your COVID-19 vaccination". www.covid19.act.gov.au. ACT Government. 11 June 2021. Retrieved 13 June 2021.
- 1 2 Bladen, Lucy; Crowe, Alex (11 September 2021). "Canberra reaches 50 per cent fully vaccinated target in over 16s". The Canberra Times. Australian Community Media. Retrieved 26 September 2021.
- ↑ "ACT UPDATE – COVID Live".
- ↑ "ATAGI statement on AstraZeneca vaccine in response to new vaccine safety concerns". health.gov.au. Department of Health. 8 April 2021. Retrieved 29 July 2021.
- ↑ "NSW temporarily suspends rollout of AstraZeneca vaccine". www.smh.com.au. Retrieved 9 April 2021.
- ↑ Xiao, Alison (10 May 2021). "Pfizer jab open to anyone in 40-49 age group as NSW mass vaccination hub opens". ABC News. Australian Broadcasting Corporation. Retrieved 11 May 2021.
- ↑ "Sydney news: Pfizer mass vaccination for HSC students, Penrith awakens to tighter COVID restrictions". ABC News. Australian Broadcasting Corporation. 9 August 2021. Retrieved 9 August 2021.
- 1 2 Vesey, Harrison (7 November 2021). "New western Sydney vaccination clinic to open at The Granville Centre". thepulse.org.au. Western Sydney Local Health District. Retrieved 4 January 2022.
- ↑ Noble, Freya (16 June 2021). "Pharmacists can now give the Astrazeneca COVID-19 vaccine in NSW". 9News. Nine Digital Pty Ltd. Retrieved 16 June 2021.
- ↑ Attanasio, Joe (12 July 2021). "AstraZeneca vaccine to become available to over-40s in NSW in desperate bid to suppress outbreak". 9 News Australia. Retrieved 13 July 2021.
- ↑ Taylor, Josh (12 July 2021). "NSW Covid update: vaccination hubs to offer over-40s AstraZeneca after 112 new cases recorded in Sydney". The Guardian. Retrieved 13 July 2021.
- 1 2 "ATAGI Statement, Response to NSW COVID-19 outbreak 24th July 2021". Department of Health. Australian Government. 24 July 2021. Retrieved 27 July 2021.
- ↑ "NSW hits vaccine milestone as Premier flags announcement about new freedom for fully vaccinated". 9News. 24 August 2021. Retrieved 24 August 2021.
- ↑ "NSW hits 70 per cent of first dose Covid-19 vaccines, signalling reopening within just weeks". Nationwide News Pty Ltd. 2 September 2021. Retrieved 2 September 2021.
- ↑ Daniel, Sue (5 September 2021). "NSW reaches vaccination milestone as peak in cases expected in next week or two". ABC News. Australian Broadcasting Corporation. Retrieved 5 September 2021.
- ↑ Daoud, Elizabeth (15 September 2021). "Major vaccine milestone achieved in NSW as 12 more COVID deaths are recorded". 7NEWS. Seven West Media. Retrieved 26 September 2021.
- ↑ "NSW reaches 50 percent of eligible population fully vaccinated". www.msn.com. Microsoft Network. 17 September 2021. Retrieved 26 September 2021.
- ↑ Parkes-Hupton, Heath. "NSW hits 60 per cent double dosed, days away from 70 per cent mark". The Australian. Nationwide News Pty Ltd. NCA NewsWire. Retrieved 26 September 2021.
- ↑ "NSW's roadmap explained: What you can and can't do once the state hits 80 and 90 per cent fully vaccinated". Retrieved 27 September 2021.
- ↑ "'YOU DID IT!' NSW officially hits 70% of eligible population fully-vaccinated". News.Com.Au. 6 October 2021.
- ↑ "NSW reaches 90 per cent first dose COVID-19 vaccination milestone". ABC News. Australian Broadcasting Corporation. 9 October 2021. Retrieved 10 October 2021.
- ↑ Brown, Natalie (8 October 2021). "Everything you can do in NSW today". News.com.au.
- ↑ "NSW hits 80 per cent double vaccination target against COVID-19". ABC News (Australia). 16 October 2021.
- ↑ "First COVID-19 vaccines administered in NT as national rollout gets underway". ABC News. Australian Broadcasting Corporation. 22 February 2021. Retrieved 11 June 2021.
- ↑ "COVID-19 Vaccine roll-out". Northern Territory PHN. 13 June 2021. Retrieved 13 June 2021.
- ↑ "Getting vaccinated". The State of Queensland. 13 June 2021. Retrieved 13 June 2021.
- ↑ Attanasio, Joe; Oliveri, Natalie (11 August 2021). "Cairns' snap lockdown ends as Queensland's first vaccination hub opens". 9 News Australia. Retrieved 11 August 2021.
- ↑ McKenna, Kate (9 August 2021). "Mass vaccination centre to open as Queensland records four cases of COVID-19 in the community". ABC News Australia. Retrieved 11 August 2021.
- ↑ "South Australian doctor receives first AstraZeneca vaccination shot in Australia". ABC News. Australian Broadcasting Corporation. 5 March 2021. Retrieved 5 March 2021.
- ↑ "Pfizer coronavirus vaccine doses wrongly delivered to Perth instead of Adelaide, delaying SA rollout". www.abc.net.au. 18 March 2021. Retrieved 9 April 2021.
- ↑ "First South Australian mass COVID-19 vaccination hub opens at Adelaide Showground, with bookings essential". ABC News. Australian Broadcasting Corporation. 30 April 2021. Retrieved 20 May 2021.
- ↑ "Book your vaccine". Department of Premier and Cabinet. 11 June 2021. Retrieved 13 June 2021.
- ↑ "Tasmania has reached a major milestone with a massive number of people over 16 now fully vaccinated". The Mercury. Hobart. Subscription required. 15 September 2021. Retrieved 26 September 2021.
- ↑ Silva, Kristian; and, Staff (20 April 2021). "Brett Sutton gets his first COVID jab as mass vaccination centres open across Victoria". ABC News. Australian Broadcasting Corporation. Retrieved 11 May 2021.
- ↑ Lawrence, Sarah (7 May 2021). "Mass COVID-19 vaccine hub to open in Bendigo CBD". ABC News - Central Victoria. Australian Broadcasting Corporation. Retrieved 20 May 2021.
- ↑ Masters, Rebecca (27 May 2021). "High-risk aged care residents don't need to wait between flu and COVID vaccine, as over 40s invited for jab in Victoria". 9 News Australia. Retrieved 30 May 2021.
- ↑ Cunningham, Melissa (27 May 2021). "Victoria opens vaccination to anyone in their 40s as COVID hotline crashes". The Age. Retrieved 30 May 2021.
- ↑ Estcourt, David (8 August 2021). "Australia's first drive-through vaccination hub to be set up in Melton, AZ jab now available to under 40s at state-run clinics". The Age. Retrieved 8 August 2021.
- ↑ "Victoria records 11 new local COVID-19 cases as Delta outbreak hits another school community". ABC News. 8 August 2021. Retrieved 8 August 2021.
- ↑ Attanasio, Joe; McPherson, Emily (8 August 2021). "Victoria launches Australia's first drive-through vaccination clinics as state records 11 new cases". 9 News. Retrieved 8 August 2021.
- ↑ "Victoria expands Pfizer vaccine access at state-run hubs to people over 16". ABC News. 24 August 2021. Retrieved 24 August 2021.
- ↑ COVID-19 Vaccine Roll-out
- ↑ "COVID-19 vaccine: Who can get vaccinated now?". Healthy WA. Department of Health, Government of Western Australia. 23 July 2021. Retrieved 23 July 2021.
- 1 2 "COVID-19 vaccine FAQs: Who is eligible for the COVID-19 vaccination?". Healthy WA. Department of Health, Government of Western Australia. 1 July 2021. Retrieved 23 July 2021.
- 1 2 "All Western Australians over 30 eligible for COVID-19 vaccinations". Government of Western Australia. 8 June 2021. Retrieved 13 June 2021.
- ↑ Perpitch, Nicolas (16 August 2021). "Pfizer COVID vaccination available for 16-29 age group in WA as border restrictions row flares up". ABC News. Retrieved 16 August 2021.
- ↑ Pearson, Nick (14 September 2021). "West Australians over 60 to be made eligible for Pfizer from Monday". 9 News Australia. Retrieved 14 September 2021.
- ↑ "Over 60s in WA eligible for Pfizer jab from next week". MSN. ABC News Australia. 14 September 2021. Retrieved 14 September 2021.
- ↑ "Provisional approval pathway: prescription medicines". Therapeutic Goods Administration. 16 February 2021. Archived from the original on 14 May 2018. Retrieved 21 February 2021.
- ↑ "TGA provisionally approves Pfizer/BioNTech COVID-19 vaccine for use in Australia". Department of Health. 25 January 2021. Archived from the original on 11 June 2021. Retrieved 26 January 2021.
- ↑ "COVID-19 vaccine: Pfizer Australia – COMIRNATY BNT162b2 (mRNA)". Therapeutic Goods Administration. 25 January 2021. Archived from the original on 9 June 2021. Retrieved 26 January 2021.
- ↑ Hitch, Georgia (7 January 2021). "When will I get the coronavirus vaccine? Who gets the vaccine first?". ABC News. Archived from the original on 11 June 2021. Retrieved 26 January 2021.
- ↑ Dye, Josh; Clun, Rachel (21 February 2021). "COVID-19 vaccines begin as Prime Minister receives Pfizer immunisation". The Sydney Morning Herald. Archived from the original on 25 May 2021. Retrieved 21 February 2021.
- ↑ "Second shipment of Pfizer COVID-19 arrives in Australia, boosting national supply". 9News. 23 February 2021. Archived from the original on 24 February 2021. Retrieved 28 February 2021.
- ↑ Worthington, Brett (9 April 2021). "Australia secures additional Pfizer vaccine following AstraZeneca concerns". ABC News. Australian Broadcasting Corporation. Archived from the original on 2 June 2021. Retrieved 24 June 2021.
- 1 2 Coughlan, Matt (17 June 2021). "AstraZeneca COVID-19 vaccine not recommended to Australians under-60". 7NEWS.com.au. AAP. Archived from the original on 24 June 2021. Retrieved 24 June 2021.
- ↑ "TGA approves Pfizer COVID-19 vaccine for 12 to 15-year-olds". Department of Health. 23 July 2021. Archived from the original on 23 July 2021. Retrieved 23 July 2021.
- ↑ "Melbourne Press Conference with Minister Hunt and Professor John Skerritt, on 5 December 2021, on the TGA provisionally approving Pfizer COVID-19 vaccine for 5 to 11-year-olds". Department of Health. 5 December 2021. Archived from the original on 7 December 2021. Retrieved 5 December 2021.
- ↑ "Australian children aged five to 11 set to receive Pfizer Covid vaccine from mid-January". The Guardian. Archived from the original on 8 December 2021. Retrieved 7 December 2021.
- ↑ "AstraZeneca COVID-19 vaccine approved for use in Australia". abc.net.au. 16 February 2021. Archived from the original on 5 June 2021. Retrieved 17 February 2021.
- ↑ "First shipment of AstraZeneca vaccine lands in Australia". 9NEWS. 28 February 2021. Archived from the original on 7 March 2021. Retrieved 28 February 2021.
- ↑ "EU, Italy stop AstraZeneca vaccine exports to Australia". 9NEWS. 5 March 2021. Retrieved 5 March 2021.
- ↑ "Australian drug regulator releases first batches of locally made AstraZeneca vaccine". The Guardian. 23 March 2021. Archived from the original on 28 March 2021. Retrieved 23 March 2021.
- ↑ Haydar, Nour (24 June 2021). "Federal government projects little need for AstraZeneca COVID-19 vaccine after October". ABC News. Australian Broadcasting Corporation. Archived from the original on 23 June 2021. Retrieved 24 June 2021.
- ↑ "TGA provisionally approves AstraZeneca's COVID-19 vaccine as booster dose". 9 February 2022. Archived from the original on 12 February 2022. Retrieved 12 February 2022.
- ↑ "TGA grants provisional determination for the Moderna COVID-19 vaccine, Elasomeran". TGA. 24 June 2021. Retrieved 1 August 2021.
- ↑ "Moderna vaccine now approved for children 12 and older". ABC News. 4 September 2021.
- 1 2 "Op COVID SHIELD National COVID Vaccine Campaign Plan". Department of Health. Australian Government. 3 August 2021. Retrieved 4 August 2021.
- ↑ "TGA grants additional provisional determination for a COVID-19 vaccine". TGA. 20 January 2021. Retrieved 1 August 2021.
- ↑ Thomas, Katie; Zimmer, Carl (24 September 2020). "Novavax Enters Final Stage of Coronavirus Vaccine Trials". The New York Times.
- ↑ "COVID-19 vaccines undergoing evaluation". 8 July 2021. Retrieved 1 August 2021..
- ↑ Rolfe, Brooke (24 January 2022). "Novavax vaccine approved: When new Covid shot will be available in Australia". News.com.au. Retrieved 26 January 2022.
- ↑ "Clinical guidance on the use of COVID-19 vaccine in Australia in 2021" (PDF). Australian Department of Health. Australian Technical Advisory Group on Immunisation (ATAGI). 17 June 2021. p. 8. Archived from the original (PDF) on 10 July 2021. Retrieved 28 July 2021.
- ↑ "AstraZeneca vaccine for people aged 18 and over". NSW Government. Retrieved 9 August 2021.
- ↑ Haydar, Nour (24 June 2021). "Federal government projects little need for AstraZeneca COVID-19 vaccine after October". ABC News. Australian Broadcasting Corporation. Retrieved 24 June 2021.
- ↑ "COVID-19 vaccines not registered in Australia but in current international use - TGA advice on "recognition"". October 2021.
- ↑ "TGA recognises two more COVID-19 vaccines not registered in Australia but used widely internationally". 1 November 2021.
- ↑ "Vaccines we recognise for the purpose of travel to Australia". Therapeutic Goods Administration. Retrieved 17 January 2022.
- ↑ "Australia's vaccine agreements". Australian Government Department of Health. 23 October 2020. Retrieved 28 January 2021.
- ↑ "Update on UQ COVID-19 vaccine - UQ News". The University of Queensland. Retrieved 28 January 2021.
- ↑ "20201211 Update on The University of Queensland COVID-19 vaccine". CSL Limited. Retrieved 28 January 2021.
- ↑ "TGA provisionally approves Pfizer/BioNTech COVID-19 vaccine for use in Australia". Australian Government Department of Health. 23 January 2021. Retrieved 28 January 2021.
- 1 2 Shields, Bevan (28 January 2021). "Vaccine supply fight escalates as EU demands millions of doses from UK". The Sydney Morning Herald. Retrieved 28 January 2021.
- ↑ "AstraZeneca COVID-19 vaccine approved for use in Australia". Australian Broadcasting Corporation. 16 February 2021. Retrieved 17 February 2021.
- 1 2 Macmillan, Jade (21 March 2021). "TGA approves domestic production of AstraZeneca's COVID-19 vaccine By political reporter". ABC News. Australian Broadcasting Corporation. Retrieved 21 March 2021.
- ↑ Clun, Rachel (28 January 2021). "AstraZeneca, Pfizer hopeful to meet vaccine supply dates despite "fluid" Europe situation". The Sydney Morning Herald. Retrieved 28 January 2021.
- 1 2 Ward, Mary (28 January 2021). "What do we know so far about Australia's COVID vaccine rollout?". The Sydney Morning Herald. Retrieved 28 January 2021.
- ↑ Marsh, Stuart (14 January 2021). "CSL rules out any local Novavax vaccine production until after AstraZeneca run". www.9news.com.au. Nine Digital Pty Ltd. Retrieved 28 January 2021.
- ↑ "AstraZeneca rejects 'completely incorrect' reports on its COVID-19 vaccine efficacy". SBS News. Agence France-Presse. 27 January 2021. Retrieved 28 January 2021.
- ↑ Davey, Melissa (27 January 2021). "'Too early' to say whether AstraZeneca Covid vaccine will go to older people in Australia". The Guardian. Retrieved 28 January 2021.
- ↑ "Expert Reaction: Scientists call for pause to AstraZeneca vaccine rollout". Scimex. 13 January 2021. Retrieved 28 January 2021.
- 1 2 Karp, Paul (28 January 2021). "Christmas Covid outbreaks a result of putting economy ahead of health, AMA says". The Guardian. Retrieved 28 January 2021.
- ↑ "Australia Secures a further 50 Million Doses of COVID-19 Vaccine" (Press release). Prime Minister of Australia. 5 November 2020. Retrieved 28 January 2021.
- ↑ Hitch, Georgia (15 February 2021). "First Pfizer coronavirus vaccine doses arrives in Australia, ahead of first jabs next week". ABC News. Australian Broadcasting Corporation. Retrieved 15 February 2021.
- ↑ Purtill, James (7 December 2020). "Distributing Pfizer and other COVID vaccines 'the largest logistics effort in the world since World War II'". ABC Health & Wellbeing. Australian Broadcasting Corporation. Retrieved 15 February 2021.
This is the largest logistics effort in the world since World War II – Dr Roberto Perez-Franco
- ↑ Antrobus, Blake (28 February 2021). "First batch of AstraZeneca vaccine arrives in Australia". NewsComAu. Nationwide News Pty Ltd. NCA NewsWire. Retrieved 28 February 2021.
- ↑ "Italy, EU refuse AstraZeneca request to ship 250,000 doses of vaccine to Australia". ABC News. Australian Broadcasting Corporation. 4 March 2021. Retrieved 5 March 2021.
- ↑ "CSL Commences Manufacturing of University of Oxford/AstraZeneca Vaccine Candidate in Melbourne". www.csl.com. CSL. 8 November 2020. Retrieved 10 August 2021.
- ↑ "The high-risk move one Australian company is hoping will pay off in the fight against COVID-19". Australian Broadcasting Corporation. ABC News. 9 November 2020. Retrieved 10 August 2021.
- ↑ Frost, Alanah (18 February 2021). "Smiles on dials with Oz vials". The Daily Telegraph. p. 5. (printed edition)
- ↑ "Seqirus to manufacture AstraZeneca COVID-19 vaccine in Australia". Manufacturer's Monthly. Retrieved 22 March 2021.
- ↑ Cunningham, Melissa; Graham, Jackson (31 March 2021). "Healthcare workers warned against 'vaccine shopping' as elderly turned away from mass clinic". The Sydney Morning Herald. Retrieved 5 April 2021.
- 1 2 Sas, Nick (4 April 2021). "Australia's COVID-19 vaccine supply is still patchy. But will other vaccines help fill the void?". ABC News. Australian Broadcasting Corporation. Retrieved 5 April 2021.
- ↑ "Human trials of potential coronavirus vaccine begin in Melbourne". ABC News. Australian Broadcasting Corporation. 25 May 2020. Retrieved 21 February 2021.
- ↑ "Australian Government urged to invest in updated vaccine manufacturing capability". Australian Academy of Science. Retrieved 5 April 2021.
- ↑ Koehn, Emma; Mannix, Liam (3 February 2021). "Scientists and government pushing for ways to make mRNA vaccines here". The Sydney Morning Herald. Retrieved 5 April 2021.
- ↑ Johnson, Paul; and, Staff (12 May 2021). "Moderna announces 25 million doses of their COVID-19 vaccine for Australia". ABC News. Australian Broadcasting Corporation. Retrieved 13 May 2021.
- ↑ "Provisional determination granted to Pfizer's COVID-19 vaccine (COMIRNATY) - proposed for use in children 5-11 years of age". 13 October 2021.
- ↑ "Australia secures an additional 20m Pfizer COVID-19 vaccines following AstraZeneca clotting concerns". ABC News. 9 April 2021.
- ↑ "Second shipment of Pfizer COVID-19 arrives in Australia, boosting national supply". 9news.com. Retrieved 27 February 2021.
- ↑ "Australian government secures 85 million Pfizer booster shots for 2022 and 2023". 9News. 25 July 2021. Retrieved 27 July 2021.
- ↑ "TGA approves booster doses of the Pfizer COVID-19 vaccine, COMIRNATY". 27 October 2021.
- ↑ "TGA provisionally approves AstraZeneca's COVID-19 vaccine as booster dose". 9 February 2022.
- ↑ "First doses of Oxford-AstraZeneca COVID vaccine arrive in Australia". abc.net.au. Retrieved 10 March 2021.
- ↑ "South Australian doctor receives first AstraZeneca vaccination shot in Australia". abc.net.au. Retrieved 5 March 2021.
- ↑ "COVID vaccine rules are changing for young children. Here's the new eligibility criteria". ABC News. 3 August 2022.
- ↑ "Moderna given final green light, available for children over six from tomorrow". ABC News. 22 February 2022.
- ↑ "TGA gives green light to Moderna vax for children aged six and older". ABC News. 17 February 2022.
- ↑ "Moderna deal is key to 'booster strategy' for Australia, Greg Hunt says". 9news.com.au. Retrieved 13 May 2021.
- ↑ "TGA provisionally approves Moderna's COVID-19 vaccine". Therapeutic Goods Administration (TGA). Australian Government Department of Health. 9 August 2021. Retrieved 18 August 2021.
- ↑ "300,000 Moderna doses on the way to Melbourne pharmacies | 7NEWS". YouTube.
- ↑ "One in five children aged 12 to 15 vaccinated as Moderna arrives". 20 September 2021.
- ↑ "TGA approves booster doses of the Moderna COVID-19 vaccine, SPIKEVAX". 8 December 2021.
- ↑ "RACGP - ATAGI rules on Novavax".
- ↑ "TGA provisionally approves Novavax (Biocelect Pty LTD's) COVID-19 vaccine NUVAXOVID". 20 January 2022.
- ↑ "Novavax is now available in Australia. Who can get it, and how much protection does it provide?". ABC News. 14 February 2022.
- ↑ "Novavax Nuvaxovid™ COVID-19 Vaccine Granted Provisional Registration in Australia for Use in Adolescents Aged 12 Through 17".
- ↑ Health, Australian Government Department of (2 March 2022). "ATAGI recommends Novavax for use as a COVID-19 booster". Australian Government Department of Health.
- ↑ "Novavax applies for provisional approval to TGA to become Australia's fourth COVID-19 vaccine". www.9news.com.au. Retrieved 1 November 2021.
- ↑ "TGA provisionally approves Novavax (Biocelect Pty Ltd's) COVID-19 vaccine NUVAXOVID". Therapeutic Goods Administration. 20 January 2022. Retrieved 20 January 2022.
- ↑ "End of December more realistic target for all Australians to get Covid vaccine, AMA says". The Guardian. 11 March 2021. Retrieved 12 March 2021.
- ↑ "Covid: Australia falls 85% short of vaccine delivery goal". BBC News. Retrieved 5 April 2021.
- ↑ "Covid-19 vaccine rollout delay hits chemists". The Australian. 6 April 2021. Retrieved 12 April 2021.
- ↑ "Fresh blow to Australia's bungled COVID-19 vaccine rollout, with pharmacists delaying coming on board". News.com.au. Retrieved 8 April 2021.
- ↑ "Why has Australia's vaccine rollout been delayed and why is it causing a fight with the EU?". Nine News. Retrieved 8 April 2021.
- ↑ "October vaccine hopes all but over after AstraZeneca blood clot concerns prompt rollout shake-up". ABC News. 8 April 2021. Retrieved 9 April 2021.
- ↑ Glenday, Jamesy; Lowrey, Tom (13 April 2021). "National cabinet to return to 'war footing' in effort to fix problem-plagued COVID vaccine rollout". ABC News. Retrieved 14 April 2021.
- ↑ "'Millions of doses': Pfizer approached Australia first for vaccine deal". 8 September 2021.
- ↑ COVID-19 Vaccine Roll-out
- ↑ McGill University. "COVID-19 Vaccine Tracker". covid19.trackvaccines.org. McGill COVID19 Vaccine Tracker Team. Retrieved 5 August 2021.
- ↑ "Trial registered on ANZCTR". anzctr.org.au. Australian New Zealand Clinical Trials Registry. Archived from the original on 27 February 2021. Retrieved 24 March 2021.
- ↑ "A phase III, Randomized, Two-armed, Double-blind, Placebo controlled trial to evaluate efficacy and safety of an adjuvanted recombinant SARS-CoV-2 spike (S) protein subunit vaccine (SpikoGen®) produced by CinnaGen Co. (Two doses of 25µg with dosing interval of 21 days)". irct.ir. 2 August 2021. IRCT20150303021315N24. Retrieved 3 August 2021.
- ↑ "INNA-051 intranasal safety and tolerability study". clinicaltrials.gov. 12 November 2021. NCT05118763. Retrieved 12 November 2021.
- ↑ "The Safety and Immunogenicity of a DNA-based Vaccine (COVIGEN) in Healthy Volunteers (COVALIA)". clinicaltrials.gov. United States National Library of Medicine. 3 February 2021.
- ↑ "Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19". clinicaltrials.gov. United States National Library of Medicine. 3 March 2021. Retrieved 21 March 2021.
- ↑ "Phase 1 Study to Assess Safety, Reactogenicity and Immunogenicity of the HDT-301 Vaccine Against COVID-19". clinicaltrials.gov. United States National Library of Medicine. Archived from the original on 12 April 2021. Retrieved 17 April 2021.
- ↑ "Icosavax Initiates Phase 1/2 Trial of COVID-19 VLP Vaccine Candidate". Icosavax. 8 June 2021. Archived from the original on 20 July 2021. Retrieved 10 June 2021.
- ↑ "A study of the safety of EDV nanocells packaged with spike-protein plasmid and glycolipid as a COVID-19 vaccine in healthy volunteers". 27 August 2021. ACTRN12621001159842. Retrieved 14 October 2021.
- ↑ "The vaccine is expected to provide long-lasting protection with a single dose administration with an anticipated safety profile similar to other licensed vaccines for active immunization" (PDF). Indian Immunologicals. 8 April 2020. Retrieved 24 July 2021.
- ↑ "'Digital certificate' for COVID-19 jab explained". Nine Digital Pty Ltd. 9 June 2021. Retrieved 9 June 2021.
- ↑ Services Australia (2 August 2021). "Add proof of your COVID-19 vaccinations to your Apple Wallet or Google Pay". Archived from the original on 4 August 2021. Retrieved 4 August 2021.
- ↑ Galloway, Anthony (13 September 2021). "Digital border pass first step to allow vaccinated Australians to come and go". Sydney Morning Herald.
- ↑ Hutchens, Gareth (2 October 2021). "What will flying look like when international borders reopen next month?". ABC News (Australia). Retrieved 2 October 2021.
- ↑ "You can get international proof of vaccinations from today — but there's a catch for those already overseas". ABC News. 19 October 2021.
- ↑ Galloway, Anthony (28 September 2021). "New app to scan QR code vaccination certificates for international travel".
- ↑ "VDS-NC Checker". 30 August 2021.
- 1 2 3 "National Cabinet sets vaccination target for path out of pandemic". Retrieved 28 July 2021.
- 1 2 "National Plan to transition Australia's National COVID-19 Response" (PDF). pm.gov.au. Australian Government. 30 July 2021. Archived from the original (PDF) on 1 August 2021. Retrieved 1 August 2021.
- ↑ "National Cabinet Statement | Prime Minister of Australia". www.pm.gov.au. 30 July 2021. Archived from the original on 5 August 2021. Retrieved 5 August 2021.
- 1 2 "Scott Morrison has announced a four-phase pathway out of Covid, so what's in Australia's pandemic exit plan?". Guardian News & Media Limited. 1 July 2021. Retrieved 28 July 2021.
- ↑ "Arriving in Australia soon? You'll no longer need to be vaccinated against COVID-19". ABC News. 3 July 2022. Retrieved 4 August 2022.
- ↑ "Australia is reopening to tourists. Here's who can travel, and when". ABC News. 8 February 2022. Retrieved 4 August 2022.
- ↑ "Doherty Institute Modelling Report to advise on the National Plan to transition Australia's National COVID Response". Department of Health. Australian Government. 3 August 2021. Retrieved 4 August 2021.
- ↑ "Coronavirus disease (COVID-19): Vaccines safety". World Health Organization. 19 February 2021. Archived from the original on 19 February 2021. Retrieved 5 August 2021.
- ↑ "Blood-clotting case in Australian AstraZeneca vaccine recipient being taken 'very seriously' by Therapeutic Goods Administration". ABC. 3 April 2021. Retrieved 5 April 2021.
- ↑ Antrobus, Blake (3 April 2021). "Coronavirus Australia: Health officer's new warning after AstraZeneca vaccine clot case". News.com.au. Retrieved 5 April 2021.
- ↑ Branco, Jorge; Pearson, Nick (16 April 2021). "TGA finds NSW woman's death 'likely' linked to AstraZeneca vaccine". 9News. Nine Digital Pty Ltd. Retrieved 16 April 2021.
- ↑ "Five new reports of blood clotting linked to AstraZeneca COVID-19 vaccine emerge in Australia, authorities investigating". ABC News. Australian Broadcasting Corporation. 6 May 2021. Retrieved 6 May 2021.
- ↑ Christmass, Pip (13 May 2021). "Seven new blood clot cases reported after AstraZeneca vaccine, TGA confirms". 7NEWS.com.au. 7 News. Retrieved 13 May 2021.
- ↑ Barnsley, Warren (18 May 2021). "Brisbane teen reportedly develops blood clots after AstraZeneca COVID-19 vaccine". 7NEWS.com.au. 7 News. Retrieved 20 May 2021.
- ↑ "SA man in 'very serious condition with blood clotting connected to AstraZeneca COVID–19 vaccine". abc.net.au. ABC News. 20 May 2021. Retrieved 20 May 2021.
- ↑ "NSW woman's blood clot death linked to AstraZeneca vaccine". 9news.com.au. 9news News. 10 June 2021. Retrieved 10 June 2021.
- 1 2 Hitch, Georgia (17 June 2021). "AstraZeneca COVID vaccine use recommended for over-60s only following ATAGI meeting". ABC News. Australian Broadcasting Corporation. Retrieved 17 June 2021.
- ↑ Gubana, Benjamin; Perpitch, Nicolas (24 June 2021). "WA Health authorities report woman's death in hospital, vaccine history sent to TGA". ABC News. Australian Broadcasting Corporation. Retrieved 12 July 2021.
- ↑ "COVID-19 vaccine weekly safety report - 08-07-2021 @ Immune thrombocytopenia (ITP)". www.tga.gov.au. Australian Government Department of Health, Therapeutic Goods Administration (TGA). 8 July 2021. Retrieved 12 July 2021.
An external Vaccine Safety Investigation Group (VSIG) of clinical experts and consumer representatives ... concluded that the woman's death was likely linked to the vaccine
- ↑ "South Australian woman dies from rare blood clots after receiving AstraZeneca vaccine". ABC News. Australian Broadcasting Corporation. 12 July 2021. Retrieved 12 July 2021.
- ↑ "TGA links deaths of 44yo Tasmanian man and 48yo Victorian woman to AstraZeneca vaccine". ABC News. Australian Broadcasting Corporation. 22 July 2021. Retrieved 22 July 2021.
- ↑ "Australian Technical Advisory Group on Immunisation (ATAGI) weekly COVID-19 meeting on 4 August 2021 update". Australian Government Department of Health. 5 August 2021. Retrieved 8 August 2021.
- ↑ "AstraZeneca vaccine 'very safe' says Chief Medical Officer, but reviews will be done". 9News. 8 April 2021. Retrieved 8 April 2021.
- ↑ "AstraZeneca vaccine: How new advice means total REPLAN of vaccine rollout". 7News. 8 April 2021. Retrieved 8 April 2021.
- ↑ "Beschluss der STIKO zur 5. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung" [Decision of the STIKO on the 5th update of the COVID-19 vaccination recommendation and the associated scientific justification]. Robert Koch Institute (in German). Federal Ministry of Health, Federal Government of Germany. 12 April 2021. Retrieved 19 May 2021.
- ↑ Morris, Loveday; Beck, Luisa (31 March 2021). "Germany halts AstraZeneca vaccine for people younger than 60 over clot concerns". The Washington Post. Retrieved 19 May 2021.
- ↑ Australian Government Department of Health (30 July 2021). "Information on COVID-19 Vaccine AstraZeneca" (PDF). Retrieved 10 August 2021.