 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
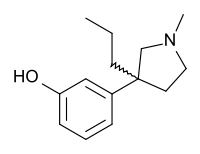
| Formula | C14H21NO |
| Molar mass | 219.328 g·mol−1 |
Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis.[1] It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic effect is 1/50 of nalorphine.[2]
Synthesis
The Knoevenagel condensation between 3'-Methoxybutyrophenone [21550-06-1] and Ethyl cyanoacetate gives (1). Conjugate addition of cyanide gives (2). Hydrolysis of both nitrile groups, saponification of the ester and decarboxylation gives the diacid, CID:164137621 (3). Imide formation occurs upon treatment with methylamine giving 3-(3-Methoxyphenyl)-1-methyl-3-propylpyrrolidine-2,5-dione, CID:163444474 (4). Reduction of the imide by lithium aluminium hydride gave [1505-32-4][29369-01-5] (5). Demethylation completed the synthesis of Profadol (6).
See also
References
- ↑ DE 1303096
- ↑ Schröder E, Rufer C, Schmiechen R (1976). Arzneimittelchemie 1. Grundlagen, Nerven, Muskeln und Gewebe. Stuttgart: Georg Thieme Verlag. ISBN 3-13-520601-7.
- ↑ Cavalla, J. F.; Jones, R.; Welford, M.; Wax, J.; Winder, C. V. (1964). "Analgetics Based on the Pyrrolidine Ring. III". Journal of Medicinal Chemistry. 7 (4): 412–415. doi:10.1021/jm00334a005.
- ↑ John Frederick Cavalla & Alan Chapman White, DE 1272296 (1968 to Parke Davis and Co LLC).
- ↑ Cavalla John Frederick, U.S. Patent 3,149,123 (1964 to Parke Davis and Co LLC).
