 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
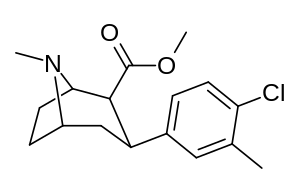
| Formula | C17H22ClNO2 |
| Molar mass | 307.82 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
RTI(-4229)-112 (2β-carbomethoxy-3β-(3-methyl-4-chlorophenyl)tropane) is a synthetic stimulant drug from the phenyltropane family. In contrast to RTI-113, which is DAT selective, RTI-112 is a nonselective triple reuptake inhibitor.[1]
In vitro tests show a very similar serotonin transporter (SERT)/dopamine transporter (DAT)/norepinephrine transporter (NET) selectivity to cocaine,[2] although in vivo behaviour is different:
"The nonselective monoamine transporter inhibitor RTI-126 and the DAT-selective inhibitors RTI-150 and RTI-336 both had a faster rate of onset (30 min) and a short duration of action (4h). In contrast, the nonselective monoamine transporter inhibitor RTI-112 had a slower rate of onset (30–60 min) and a longer duration of action (10h). The DAT-selective inhibitors RTI-171 and RTI-177 also had slower rates of onset (30–120 min), but RTI-171 had a short duration of action (2.5 h) while RTI-177 had a very long duration of action (20 h)."[3]
The efficacy of cocaine analogs to elicit self-administration is related to the rate at which they are administered. Slower onset analogs are less likely to function as behavioral stimulants than analogs eliciting a faster rate of onset.[4] Nonselective analogs are less likely to function as "reinforcers" than reuptake inhibitors that have DAT specificity.[3]
In order for a dopamine reuptake inhibitor (DRI) such as cocaine to induce euphoria, PET scans on primates reveal that the DAT occupancy needs to be >60%.[5]
RTI-112 has equipotent in vitro affinity at the SERT, NET and DAT, respectively.[2] RTI-112 was not reliably self-administered, in contrast to the DAT selective reuptake inhibitors that were used in this study.[2]
In vivo at the ED50, RTI-112 had no DAT occupancy at all.[2] At the ED50, almost all of the RTI-112 occupied the SERT at this dose.[2] A significantly higher dose was required to get >70% DAT occupancy in the case of RTI-112;[2] however, RTI-112 was still able to suppress cocaine administration at the ED50, suggesting a serotonergic mechanism was responsible for this.[2]
References
- ↑ Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL (March 2005). "Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys" (PDF). Pharmacology, Biochemistry, and Behavior. 80 (3): 481–491. doi:10.1016/j.pbb.2005.01.004. PMID 15740791. S2CID 10004289. Archived from the original (PDF) on 2010-06-11.
- 1 2 3 4 5 6 7 Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. (June 2004). "Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 309 (3): 959–969. doi:10.1124/jpet.103.060293. PMID 14982963. S2CID 39794215. Archived from the original (PDF) on 2010-06-11. Retrieved 2009-07-15.
- 1 2 Kimmel HL, O'Connor JA, Carroll FI, Howell LL (January 2007). "Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys". Pharmacology, Biochemistry, and Behavior. 86 (1): 45–54. doi:10.1016/j.pbb.2006.12.006. PMC 1850383. PMID 17258302.
- ↑ Wee S, Carroll FI, Woolverton WL (February 2006). "A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants". Neuropsychopharmacology. 31 (2): 351–362. doi:10.1038/sj.npp.1300795. PMID 15957006.
- ↑ Howell LL, Wilcox KM (July 2001). "The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 298 (1): 1–6. PMID 11408518. Archived from the original (PDF) on 2006-09-21.