 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
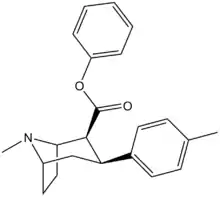
| Formula | C22H25NO2 |
| Molar mass | 335.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
(–)-2β-Carbophenoxy-3β-(p-tolyl)tropane (RTI-4229-120) is a phenyltropane derivative which acts as a reasonably selective dopamine reuptake inhibitor, along with weaker inhibition of noradrenaline and serotonin reuptake.[1] It has a reasonably fast rate of occupancy of dopamine transporters in the brain, though slower than that of cocaine itself.[2] RTI-120 has a short duration of action, along with other p-methyl substituted phenyltropanes such as RTI-150, RTI-171 and RTI-199, giving it a more similar pharmacological profile to cocaine compared to longer acting analogues like RTI-121 and RTI-177.[3]
See also
References
- ↑ Silverthorn ML, Dersch CM, Baumann MH, Cadet JL, Partilla JS, Rice KC, Carroll FI, Becketts KM, Brockington A, Rothman RB (April 1995). "Studies of the biogenic amine transporters. V. Demonstration of two binding sites for the cocaine analog [125I]RTI-55 associated with the 5-HT transporter in rat brain membranes". The Journal of Pharmacology and Experimental Therapeutics. 273 (1): 213–22. PMID 7714769.
- ↑ Stathis M, Scheffel U, Lever SZ, Boja JW, Carroll FI, Kuhar MJ (June 1995). "Rate of binding of various inhibitors at the dopamine transporter in vivo" (PDF). Psychopharmacology. 119 (4): 376–84. doi:10.1007/BF02245852. PMID 7480516.
- ↑ Kimmel HL, Carroll FI, Kuhar MJ (December 2001). "Locomotor stimulant effects of novel phenyltropanes in the mouse". Drug and Alcohol Dependence. 65 (1): 25–36. doi:10.1016/S0376-8716(01)00144-2. PMID 11714587.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.