 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H18N2 |
| Molar mass | 202.301 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
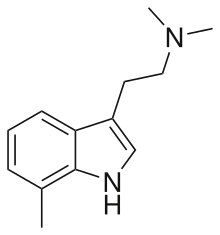
7,N,N-trimethyltryptamine (7-methyl-DMT, 7-TMT), is a tryptamine derivative which acts as an agonist of 5-HT2 receptors.[1][2][3] In animal tests, both 7-TMT and its 5-methoxy derivative 5-MeO-7-TMT produced behavioural responses similar to those of psychedelic drugs such as DMT, but the larger 7-ethyl and 7-bromo derivatives of DMT did not produce psychedelic responses despite having higher 5-HT2 receptor affinity in vitro (cf. DOBU, DOAM).[4] 7-TMT also weakly inhibits reuptake of serotonin but with little effect on dopamine or noradrenaline reuptake.[5]
See also
References
- ↑ Glennon RA, Liebowitz SM, Mack EC (August 1978). "Serotonin receptor binding affinities of several hallucinogenic phenylalkylamine and N,N-dimethyltryptamine analogues". Journal of Medicinal Chemistry. 21 (8): 822–5. doi:10.1021/jm00206a022. PMID 278843.
- ↑ Glennon RA, Gessner PK (April 1979). "Serotonin receptor binding affinities of tryptamine analogues". Journal of Medicinal Chemistry. 22 (4): 428–32. doi:10.1021/jm00190a014. PMID 430481.
- ↑ Lyon RA, Titeler M, Seggel MR, Glennon RA (January 1988). "Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens". European Journal of Pharmacology. 145 (3): 291–7. doi:10.1016/0014-2999(88)90432-3. PMID 3350047.
- ↑ Glennon RA, Schubert E, Jacyno JM, Rosecrans JA (November 1980). "Studies on several 7-substituted N,N-dimethyltryptamines". Journal of Medicinal Chemistry. 23 (11): 1222–6. doi:10.1021/jm00185a014. PMID 6779006.
- ↑ Glennon RA, Martin B, Johnson KM, End D (January 1978). "7,N,N-Trimethyltryptamine: a selective inhibitor of synaptosomal serotonin uptake". Research Communications in Chemical Pathology and Pharmacology. 19 (1): 161–4. PMID 625585.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.