 | |
 | |
| Names | |
|---|---|
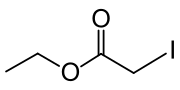
| Preferred IUPAC name
Ethyl iodoacetate | |
| Other names
Ethyl 2-iodoacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.816 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
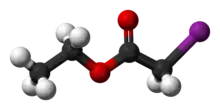
| C4H7IO2 | |
| Molar mass | 214.002 g·mol−1 |
| Density | 1.808 g/mL |
| Boiling point | 179 to 180 °C (354 to 356 °F; 452 to 453 K) |
| -97.6·10−6 cm3/mol | |
| Hazards | |
| GHS labelling:[1] | |
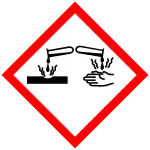  | |
| Danger | |
| H300, H314 | |
| P280, P301+P310+P330, P301+P330+P331, P303+P361+P353, P305+P351+P338+P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ethyl iodoacetate is a chemical compound that is a derivative of ethyl acetate.[2][3] Under normal conditions, the compound is a clear, light yellow to orange liquid.
Applications
Used by the British during World War I, it was codenamed SK gas, for the initials of South Kensington, where it was developed.[4]
Like many alkyl iodides, ethyl iodoacetate is an alkylating agent, which makes it useful in organic synthesis, yet toxic. Ethyl iodoacetate is also a lachrymatory agent.
References
- ↑ GHS: Sigma-Aldrich 242934
- ↑ "242934 ALDRICH Ethyl iodoacetate". Sigma Aldrich. sigmaaldrich.com. Retrieved 1 June 2017.
- ↑ "Ethyl iodoacetate". chemicalbook.com. Retrieved 1 June 2017.
- ↑ Timothy T. Marrs; Robert L. Maynard; Frederick Sidell (4 April 2007). Chemical Warfare Agents: Toxicology and Treatment. John Wiley & Sons. pp. 682–. ISBN 978-0-470-06002-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.