 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
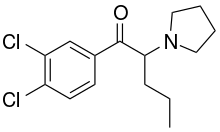
| Formula | C15H19Cl2NO |
| Molar mass | 300.22 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
O-2390 (3,4-Dichloro-alpha-PVP, DCPVP) is a recreational designer drug from the substituted cathinone family, which acts as a potent inhibitor of dopamine and noradrenaline reuptake in vitro, with weaker but still significant inhibition of serotonin reuptake.[1][2][3][4]
See also
References
- ↑ Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors". Journal of Medicinal Chemistry. 49 (4): 1420–1432. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
- ↑ Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA (October 2015). "Structural Modification of the Designer Stimulant α-Pyrrolidinovalerophenone (α-PVP) Influences Potency at Dopamine Transporters". ACS Chemical Neuroscience. 6 (10): 1726–1731. doi:10.1021/acschemneuro.5b00160. PMC 5349767. PMID 26217965.
- ↑ Glennon RA, Young R (September 2016). "Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP)". Brain Research Bulletin. 126 (Pt 1): 111–126. doi:10.1016/j.brainresbull.2016.04.011. PMC 5817884. PMID 27142261.
- ↑ Yadav-Samudrala BJ, Eltit JM, Glennon RA (September 2019). "Synthetic Cathinone Analogues Structurally Related to the Central Stimulant Methylphenidate as Dopamine Reuptake Inhibitors". ACS Chemical Neuroscience. 10 (9): 4043–4050. doi:10.1021/acschemneuro.9b00284. PMID 31369229. S2CID 199380459.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.