 | |
| Clinical data | |
|---|---|
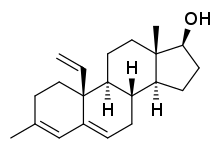
| Other names | 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H30O |
| Molar mass | 298.470 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol is a synthetic, steroidal estrogen and a selective agonist of the ERβ.[1] It was discovered serendipitously and was the lead compound among a series of androsta-3,5-dienes as ERβ ligands.[1][2] Its affinity (IC50) for the ERβ was found to be 9 nM and it showed 62- and 160-fold binding selectivity for this receptor over the AR and the ERα, respectively.[1] The EC50 of the compound for the ERβ was found to be 69 nM and its intrinsic activity was 92% (relative to that of estradiol).[1] As such, it is a potent ERβ agonist with high affinity and selectivity.[1]
References
- 1 2 3 4 5 Blizzard TA, Gude C, Morgan JD, Chan W, Birzin ET, Mojena M, Tudela C, Chen F, Knecht K, Su Q, Kraker B, Mosley RT, Holmes MA, Rohrer SP, Hammond ML (2007). "Androstene-3,5-dienes as ER-beta selective SERMs". Bioorg. Med. Chem. Lett. 17 (22): 6295–8. doi:10.1016/j.bmcl.2007.09.001. PMID 17890084.
- ↑ Khalil RA (2013). "Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease". Biochem. Pharmacol. 86 (12): 1627–42. doi:10.1016/j.bcp.2013.09.024. PMC 3840081. PMID 24099797.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.