 | |
| Clinical data | |
|---|---|
| Other names | CB-7432, SB-223030; Pyrrolidino-4-iodotamoxifen; 4-Iodopyrrolidinotamoxifen |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Elimination half-life | Acute: 15 hours[1] Chronic: 23 days[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
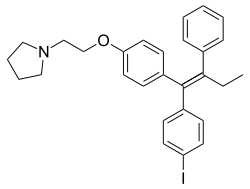
| Formula | C28H30INO |
| Molar mass | 523.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Idoxifene (INN, USAN, BAN) (former developmental code names CB-7432, SB-223030), also known as pyrrolidino-4-iodotamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group which was under development for the treatment of breast cancer and postmenopausal osteoporosis but was never marketed.[1][2][3] It reached phase III clinical trials for postmenopausal osteoporosis and phase II clinical trials for breast cancer before development was discontinued in 1999 due to insufficient effectiveness in both cases.[1]
Chemistry
Synthesis
A large-scale chemical synthesis of idoxifene has been devised.[4]
References
- 1 2 3 4 "Idoxifene". AdisInsight. Springer Nature Switzerland AG.
- ↑ Miller WR, Ingle JN (8 March 2002). Endocrine Therapy in Breast Cancer. CRC Press. pp. 58–. ISBN 978-0-203-90983-6.
- ↑ McCague R, Leclercq G, Legros N, Goodman J, Blackburn GM, Jarman M, Foster AB (December 1989). "Derivatives of tamoxifen. Dependence of antiestrogenicity on the 4-substituent". Journal of Medicinal Chemistry. 32 (12): 2527–2533. doi:10.1021/jm00132a006. PMID 2585441.
- ↑ McCague R, Potter GA, Jarman M (1994). "An Efficient, Large‐Scale Synthesis of Idoxifene ((E)‐1‐(4‐(2‐(N‐Pyrrolidino) ethoxy) phenyl)‐1‐(4‐Iodophenyl)‐2‐phenyl‐1‐butene)". Organic Preparations and Procedures International. 26 (3): 343–346. doi:10.1080/00304949409458432. ISSN 0030-4948.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.