 | |
| Names | |
|---|---|
| IUPAC name
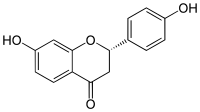
(2S)-4′,7-Dihydroxyflavan-4-one | |
| Systematic IUPAC name
(2S)-7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O4 | |
| Molar mass | 256.257 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Liquiritigenin is a flavanone that was isolated from Glycyrrhiza uralensis, and is found in a variety of plants of the Glycyrrhiza genus, including Glycyrrhiza glabra (licorice).[1] It is an estrogenic compound which acts as a selective agonist of the ERβ subtype of the estrogen receptor (ER),[2] though it is also reported to act as an ERα partial agonist at sufficient concentrations.[3] It also has a choleretic effect.[1]
Liquiritigenin,NADPH:oxygen oxidoreductase (hydroxylating, aryl migration) is an enzyme that uses liquiritigenin, O2, NADPH and H+ to produce 2,7,4'-trihydroxyisoflavanone, H2O, and NADP+.
See also
- Menerba
- Prinaberel (ERB-041)
- Diarylpropionitrile (DPN)
- WAY-200070
- PHTPP
- (R,R)-Tetrahydrochrysene ((R,R)-THC)
- Propylpyrazoletriol (PPT)
- Methylpiperidinopyrazole (MPP)
References
- 1 2 Kim, YW; Kang, HE; Lee, MG; Hwang, SJ; Kim, SC; Lee, CH; Kim, SG (2009). "Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes". American Journal of Physiology. Gastrointestinal and Liver Physiology. 296 (2): G372–81. doi:10.1152/ajpgi.90524.2008. PMID 19074639.
- ↑ Mersereau, Jennifer E.; Levy, Nitzan; Staub, Richard E.; Baggett, Scott; Zogric, Tetjana; Chow, Sylvia; Ricke, William A.; Tagliaferri, Mary; et al. (2008). "Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist". Molecular and Cellular Endocrinology. 283 (1–2): 49–57. doi:10.1016/j.mce.2007.11.020. PMC 2277338. PMID 18177995.
- ↑ Green, Sarah E (2015), In Vitro Comparison of Estrogenic Activities of Popular Women's Health Botanicals, archived from the original on 2016-02-22, retrieved 2016-01-01
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.