 | |
| Clinical data | |
|---|---|
| Trade names | Pontocaine, Ametop, Dicaine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682640 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 75.6 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.106 |
| Chemical and physical data | |
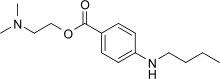
| Formula | C15H24N2O2 |
| Molar mass | 264.369 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tetracaine, also known as amethocaine, is an ester local anesthetic used to numb the eyes, nose, or throat.[2] It may also be applied to the skin before starting an intravenous (injection) to decrease pain from the procedure.[3] Typically it is applied as a liquid to the area.[2] Onset of effects when used in the eyes is within 30 seconds and last for less than 15 minutes.[2]
Common side effects include a brief period of burning at the site of use.[2] Allergic reactions may uncommonly occur.[4] Long-term use is generally not recommended as it may slow healing of the eye.[2] It is unclear if use during pregnancy is safe for the baby.[2] Tetracaine is in the ester-type local anesthetic family of medications.[4] It works by blocking the sending of nerve impulses.[2]
Tetracaine was patented in 1930 and came into medical use in 1941.[5] It is on the World Health Organization's List of Essential Medicines[6]
Medical uses
A systematic review investigated tetracaine for use in emergency departments, especially for starting intravenous lines in children, in view of its analgesic and cost-saving properties. However, it did not find an improvement in first-attempt cannulations.[7]
Tetracaine is the T in TAC, a mixture of 5 to 12% tetracaine, 0.05% adrenaline, and 4 or 10% cocaine hydrochloride used in ear, nose, and throat surgery and in the emergency department where numbing of the surface is needed rapidly, especially when children have been injured in the eye, ear, or other sensitive locations.[8]
Mechanism
In biomedical research, tetracaine is used to alter the function of calcium release channels (ryanodine receptors) that control the release of calcium from intracellular stores. Tetracaine is an allosteric blocker of channel function. At low concentrations, tetracaine causes an initial inhibition of spontaneous calcium release events, while at high concentrations, tetracaine blocks release completely.[9]
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- 1 2 3 4 5 6 7 "Tetracaine". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 769, 897. ISBN 9780857111562.
- 1 2 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 437. hdl:10665/44053. ISBN 9789241547659.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 475. ISBN 9783527607495. Archived from the original on 2016-12-29.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Pywell A, Xyrichis A (September 2015). "Does topical Amethocaine cream increase first-time successful cannulation in children compared with a eutectic mixture of local anaesthetics (EMLA) cream? A systematic review and meta-analysis of randomised controlled trials". Emergency Medicine Journal. Emerg Med J. 32 (9): 733–737. doi:10.1136/emermed-2014-204066. PMID 25351196. S2CID 21769195.
- ↑ Appleton's Nursing Manual - "Cocaine"
- ↑ Györke S, Lukyanenko V, Györke I (April 1997). "Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes". The Journal of Physiology. 500 (Pt 2): 297–309. doi:10.1113/jphysiol.1997.sp022021. PMC 1159384. PMID 9147318.
External links
- "Tetracaine". Drug Information Portal. U.S. National Library of Medicine.
- "Tetracaine hydrochloride". Drug Information Portal. U.S. National Library of Medicine.