| |
| Clinical data | |
|---|---|
| Trade names | Tonocard |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a601248 |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 0.9-1 (oral) |
| Protein binding | 10-20% |
| Metabolism | glucuronidation (primary) |
| Elimination half-life | 9-14 R, 13-20 S |
| Excretion | 30-50% urine (unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.441 |
| Chemical and physical data | |
| Formula | C11H16N2O |
| Molar mass | 192.262 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tocainide (Tonocard) is a class Ib antiarrhythmic agent. It is no longer sold in the United States.
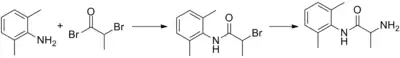
Synthesis
Pharmacokinetics
Tocainide is a lidocaine derivative, that undergoes very less first pass metabolism. It occurs as two enantiomers. The R isomer is three times more potent than the S isomer.[5] Tocainide's oral bioavailability is almost 100%.[6] Plasma half-life generally lasts for 11.5-15.5 hours (13.5 ± 2 hours[7]). In the blood, tocainide is 10-20% protein bound.[8][6] The volume of distribution is 2.8-3.2 L/kg.[8] 31-45% is excreted unchanged in the urine.[8] The more active R-isomer is cleared faster in anephric patients (without kidneys) or those with severe kidney dysfunction. The main metabolite is tocainide carbamoyl ester glucuronlde.[9]
Drug interactions
Rifampicin increases conversion of tocainide into its main metabolite, tocainide carbamoyl ester glucuronlde,[9] by inducing the glucuronosyl transferase enzyme that catalyzes glucuronidation of tocainide to produce that metabolite. Rifampicin also increases elimination rate and decreases oral clearance of tocainide.[10] Tocainide decreases plasma clearance of theophylline.[11]
References
- ↑ DE 2235745, Boyes RN, Byrnes EW, "Antiarrhythmisch Wirksame Verbindung, Verfahren zu Deren Herstellung und Deren Verwendung", issued 1972, assigned to Astra Pharmaceutical Products Inc.
- ↑ GB 1461602, "Primary Amino Acylanilides Methods of Making the Same and Use as Antiarrhythmic Drugs", issued 1974, assigned to Astra Pharmaceutical Products Inc.
- ↑ DE 2400540, Boyes RN, Duce BR, Smith EM, Byrnes EW, "Primaeraminoacylanilide, Verfahren zu Deren Herstellung und Sie Enthaltende Arzneimittel", issued 1974, assigned to Astra Pharmaceutical Products Inc.
- ↑ Byrnes EW, McMaster PD, Smith ER, Blair MR, Boyes RN, Duce BR, et al. (October 1979). "New antiarrhythmic agents. 1. Primary alpha-amino anilides". Journal of Medicinal Chemistry. 22 (10): 1171–6. doi:10.1021/jm00196a005. PMID 513064.
- ↑ Tricarico, D.; Fakler, B.; Spittelmeister, W.; Ruppersberg, J. P.; Stützel, R.; Franchini, C.; Tortorella, V.; Conte-Camerino, D.; Rüdel, R. (1991-04-01). "Stereoselective interaction of tocainide and its chiral analogs with the sodium channels in human myoballs". Pflügers Archiv. 418 (3): 234–237. doi:10.1007/BF00370521. ISSN 1432-2013. PMID 1649990. S2CID 24456292.
- 1 2 Kutalek, S. P.; Morganroth, J.; Horowitz, L. N. (September 1985). "Tocainide: a new oral antiarrhythmic agent". Annals of Internal Medicine. 103 (3): 387–391. doi:10.7326/0003-4819-103-3-387. ISSN 0003-4819. PMID 3927807.
- ↑ Winkle, R A; Meffin, P J; Fitzgerald, J W; Harrison, D C (December 1976). "Clinical efficacy and pharmacokinetics of a new orally effective antiarrhythmic, tocainide". Circulation. 54 (6): 885–889. doi:10.1161/01.CIR.54.6.885. ISSN 0009-7322. PMID 791536.
- 1 2 3 "Kidney Disease Program (KDP)". University of Louisville. Archived from the original on 2023-12-12. Retrieved 2023-12-12.
- 1 2 Kwok, David W. K. (1987). Studies on the metabolism of tocainide in humans (Thesis). University of British Columbia.
- ↑ Rice, T. L.; Patterson, J. H.; Celestin, C.; Foster, J. R.; Powell, J. R. (March 1989). "Influence of rifampin on tocainide pharmacokinetics in humans". Clinical Pharmacy. 8 (3): 200–205. ISSN 0278-2677. PMID 2495879.
- ↑ Loi, C. M.; Wei, X.; Parker, B. M.; Korrapati, M. R.; Vestal, R. E. (April 1993). "The effect of tocainide on theophylline metabolism". British Journal of Clinical Pharmacology. 35 (4): 437–440. doi:10.1111/j.1365-2125.1993.tb04163.x. ISSN 0306-5251. PMC 1381557. PMID 8485025.
Further reading
- Burton, Michael E. (2006). Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. Lippincott Williams & Wilkins. ISBN 9780781744317. OCLC 59148565.
External links