 | |
| Names | |
|---|---|
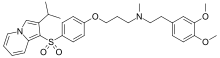
| Preferred IUPAC name
N-[2-(3,4-Dimethoxyphenyl)ethyl]-N-methyl-3-{4-[2-(propan-2-yl)indolizine-1-sulfonyl]phenoxy}propan-1-amine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C31H38N2O5S | |
| Molar mass | 550.71 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Fantofarone is a calcium channel blocker.[1]
Comparison with verapamil
There are many different calcium channel blockers that show different results in use. Fantofarone and verapamil are both calcium channel blockers that behave differently in different applications.
Fantofarone was shown in use for treatment of angioplasty-induced vasospasm in an atherosclerotic rabbits.[2] There are many different observations that show a potential cause of vasospasm, such as local injury on the body, but overall mechanism of it is still not fully understood.[2] In order to observe the efficiency of fantofarone in the treatment of angioplasty-induced vasospasm (AIV), it was compared to the treatment of verapamil (which is also a calcium channel blocker). Fantofarone showed more effectiveness against a severity of vasospasm than verapamil.[2]
Fantofarone and verapamil were also tested as an attempt of reversing resistance to chloroquine,[3] a medication that is used to treat malaria. In the treatment of reversing the chloroquine resistance it was observed that verapamil was more potent and more efficient than fantofarone.[3]
References
- ↑ Rosseels, Gilbert; Houben, Christian; Kerckx, Patricia (1995). "Synthesis of a metabolite of fantofarone". Advances in Organobromine Chemistry II. Industrial Chemistry Library. Vol. 7. pp. 152–159. doi:10.1016/S0926-9614(05)80016-4. ISBN 9780444821058.
- 1 2 3 Dongay, Bruno; Dol-Gleizes, Frédérique; Herbert, Jean-Marc (1998-06-15). "Effect of Fantofarone, a New Ca2 Channel Antagonist, on Angioplasty-Induced Vasospasm in an Atherosclerotic Rabbit Model". Biochemical Pharmacology. 55 (12): 2047–2050. doi:10.1016/S0006-2952(98)00026-4. ISSN 0006-2952. PMID 9714327.
- 1 2 Adovelande, Jacques; Delèze, Jean; Schrével, Joseph (1998-02-15). "Synergy between two calcium channel blockers, verapamil and fantofarone (SR33557), in reversing chloroquine resistance in Plasmodium falciparum". Biochemical Pharmacology. 55 (4): 433–440. doi:10.1016/S0006-2952(97)00482-6. ISSN 0006-2952. PMID 9514077.