 | |
| Clinical data | |
|---|---|
| Trade names | Menformon Prolongatum, Dimenformon Prolongatum, Lynandron Prolongatum |
| Other names | Estradiol phenpropionate; Estradiol benzenepropionate; Estradiol phenylpropanoate; Estradiol benzenepropanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.340 |
| Chemical and physical data | |
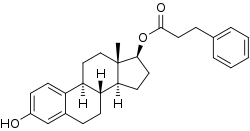
| Formula | C27H32O3 |
| Molar mass | 404.550 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estradiol phenylpropionate (EPP), also known as estradiol 17β-phenylpropionate and sold under the brand name Menformon Prolongatum, is an estrogen which is no longer marketed.[1][2][3] It is an estrogen ester, specifically the C17β phenylpropionate ester of estradiol.[1]
EPP has been marketed in combination with estradiol benzoate under the brand name Dimenformon Prolongatum in Europe and in combination with estradiol benzoate, testosterone propionate, testosterone phenylpropionate, and testosterone isocaproate under the brand names Mixogen, Estandron Prolongatum, and Lynandron Prolongatum (a balanced mixture of estradiol and testosterone esters) in menopausal hormone therapy.[4][1][2][5][3][6][7] Both of these medication combinations are long-acting injectables indicated in hormone replacement therapy for women in menopause. Dimenformon Prolongatum has also been investigated as a single injection, "morning after" post-coital contraceptive, and is additionally used as a component of hormone replacement therapy for transgender women.[2]
The pharmacokinetics of EPP in combination with estradiol benzoate have been studied.[8][9]
See also
References
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 406–. ISBN 978-3-88763-075-1.
- 1 2 3 Garaffa G, Ralph DJ (12 February 2015). "Female to Male Transsexualism". In Mirone V (ed.). Clinical Uro-Andrology. Springer. pp. 17–. ISBN 978-3-662-45018-5.
- 1 2 Hager HH, Kern W, List PH, Roth HJ (29 July 2013). "Oestradiol". Hagers Handbuch der Pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Ärzte und Medizinalbeamte: Wirkstoffgruppen II Chemikalien und Drogen (A-AL). Springer-Verlag. pp. 157–. ISBN 978-3-662-25655-8.
- ↑ Saure A (1989). "Hormonpräparate, die zur Behandlung der Wechseljahre eingesetzt werden". Die Wechseljahre der Frau. pp. 153–164. doi:10.1007/978-3-0348-6676-7_11. ISBN 978-3-7643-1947-2.
- ↑ "MIXOGEN® (Injection)". Archived from the original on 2016-05-30. Retrieved 2016-06-10.
- ↑ Pschyrembeln W (15 June 2011). "Störungen in der Postmenopause". Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 533–. ISBN 978-3-11-150424-7.
- ↑ Rote Liste: Verzeichnis pharmazeutischer Spezialpräparate. Editio Cantor. 1965.
Lynandron Prolongatum ® (Organon -> O 70) Zus. 1 Ampulle zu 1 ml: 1 mg Oestradiolbenzoat, 4 mg Oestradiolphenylpropionat, 20 mg Testosteronpropionat, 40 mg Testosteronphenylpropionat, 40 mg Testosteronisocapronat.
- ↑ Rauramo L, Punnonen R, Kaihola LH, Grönroos M (January 1980). "Serum oestrone, oestradiol and oestriol concentrations in castrated women during intramuscular oestradiol valerate and oestradiolbenzoate-oestradiolphenylpropionate therapy". Maturitas. 2 (1): 53–58. doi:10.1016/0378-5122(80)90060-2. PMID 7402086.
- ↑ Rauramo L, Punnonen R, Grönroos M (August 1981). "Serum concentrations of oestrone, oestradiol and oestriol during various oestrogen treatments". Maturitas. 3 (2): 183–186. doi:10.1016/0378-5122(81)90010-4. PMID 7289888.