Dihydropyridine calcium channel blockers are derivatives of 1,4-dihydropyridine that are used as L-type calcium channel blockers.[1] They are used in the treatment of hypertension.[2]
Class members
Compared with certain other L-type calcium channel blockers (for example those of the phenylalkylamine class such as verapamil) that have significant action at the heart, they are relatively vascular selective in their mechanism of action in lowering blood pressure.[3]
Dihydropyridine class L-type calcium channel blockers include, in alphabetical order (brand names vary in different countries):
| Name | Image | Brand name | Citations |
| Amlodipine | 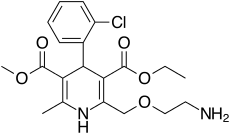 | Norvasc, Istin, Normodipine, Tenox, Cordi Cor | [4] |
| Aranidipine |  | Sapresta (サプレスタ) | [5] |
| Azelnidipine |  | CalBlock (カルブロック) | [6] |
| Barnidipine |  | Vasexten, Libradin, Cyress, HypoCa | [7] |
| Benidipine |  | Coniel | [8] |
| Cilnidipine |  | Atelec (アテレック), Cilacar, Cinalong, Siscard | [9] |
| Clevidipine |  | Cleviprex | [10] |
| Cronidipine |  | [11] | |
| Darodipine |  | [12] | |
| Dexniguldipine |  | [13] | |
| Efonidipine |  | Landel (ランデル) | [14] |
| Elgodipine |  | [15] | |
| Elnadipine |  | [16] | |
| Felodipine |  | Renedil, Plendil | [17] |
| Flordipine |  | [18] | |
| Furnidipine |  | [19] | |
| Iganidipine |  | [20] | |
| Isradipine |  | DynaCirc CR | [21] |
| Lacidipine |  | Lacipil, Motens, Sakure | |
| Lemildipine |  | ||
| Lercanidipine |  | Zanidip, Zanidip-Recordati | |
| Levamlodipine | 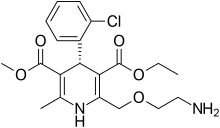 | EsCordi Cor | |
| Levniguldipine |  | ||
| Manidipine |  | Manyper, Caslot, Madipine | |
| Nicardipine |  | Cardene, Cardene SR | |
| Nifedipine |  | Adalat, Nifedical, Procardia, Corinfar, Cordaflex | |
| Niguldipine |  | ||
| Niludipine |  | ||
| Nilvadipine |  | Nivadil | |
| Nimodipine | 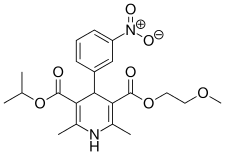 | Nimotop | |
| Nisoldipine |  | Sular, Baymycard, Syscor | |
| Nitrendipine |  | Baypress, Cardif, Nitrepin, Baylotensin | |
| Olradipine |  | ||
| Oxodipine |  | ||
| Palonidipine |  | ||
| Pranidipine |  | Acalas | |
| Ryodipine | 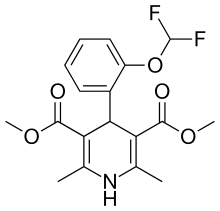 | ||
| Sagandipine |  | ||
| Sornidipine |  | ||
| Teludipine |  | ||
| Tiamdipine |  | ||
| Trombodipine |  | ||
| Vatanidipine |  | ||
The pharmaceutical drug finerenone is also a dihydrophyridine derivative, but does not act as a calcium channel blocker but as an antimineralocorticoid.[22]
See also
- Calcium channel blocker (including section on non-dihydropyridine calcium channel blockers)
- Calcium channel
- Dihydropyridine receptor
References
- ↑ Bladen, Chris; Gündüz, Miyase Gözde; Şimşek, Rahime; Şafak, Cihat; Zamponi, Gerald W. (2014-10-23). "Synthesis and evaluation of 1,4-dihydropyridine derivatives with calcium channel blocking activity". Pflügers Archiv: European Journal of Physiology. 466 (7): 1355–1363. doi:10.1007/s00424-013-1376-z. ISSN 1432-2013. PMID 24149495. S2CID 253888496.
- ↑ Xu, Lei; Li, Dan; Tao, Li; Yang, Yanling; Li, Youyong; Hou, Tingjun (2016). "Binding mechanisms of 1,4-dihydropyridine derivatives to L-type calcium channel Cav1.2: a molecular modeling study". Molecular BioSystems. 12 (2): 379–390. doi:10.1039/c5mb00781j. ISSN 1742-2051. PMID 26673131.
- ↑ Frishman, William H. (2007). "Calcium channel blockers: differences between subclasses". American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions. 7 (Suppl 1): 17–23. doi:10.2165/00129784-200707001-00003. ISSN 1175-3277. PMID 19845073. S2CID 21199295.
- ↑ Bulsara, Kishen G.; Cassagnol, Manouchkathe (2022), "Amlodipine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30137793, retrieved 2023-01-05
- ↑ Okumura, K.; Ichihara, K.; Nagasaka, M. (1997). "Effects of aranidipine, a novel calcium channel blocker, on mechanical responses of the isolated rat portal vein: comparison with typical calcium channel blockers and potassium channel openers". Journal of Cardiovascular Pharmacology. 29 (2): 209–215. doi:10.1097/00005344-199702000-00009. ISSN 0160-2446. PMID 9057070.
- ↑ Wellington, Keri; Scott, Lesley J. (2003). "Azelnidipine". Drugs. 63 (23): 2613–2621, discussion 2623–2624. doi:10.2165/00003495-200363230-00004. ISSN 0012-6667. PMID 14636080. S2CID 263994294.
- ↑ Malhotra, H. S.; Plosker, G. L. (2001). "Barnidipine". Drugs. 61 (7): 989–996, discussion 997–998. doi:10.2165/00003495-200161070-00007. ISSN 0012-6667. PMID 11434453. S2CID 263999687.
- ↑ Opie, LIONEL H. (2013-01-01), Opie, Lionel H.; Gersh, Bernard J. (eds.), "3 - Calcium channel blockers", Drugs for the Heart (Eighth Edition), Philadelphia: W.B. Saunders, pp. 64–92, ISBN 978-1-4557-3322-4, retrieved 2023-01-05
- ↑ Shete, Mukesh Madhukar (2016). "Cilnidipine: Next Generation Calcium Channel Blocker". The Journal of the Association of Physicians of India. 64 (4): 95–99. ISSN 0004-5772. PMID 27734656.
- ↑ Deeks, Emma D.; Keating, Gillian M.; Keam, Susan J. (2009). "Clevidipine: a review of its use in the management of acute hypertension". American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions. 9 (2): 117–134. doi:10.1007/BF03256583. ISSN 1175-3277. PMID 19331440.
- ↑ PubChem. "Cronidipine". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-01-05.
- ↑ Matucci, R.; Ottaviani, M. F.; Barbieri, M.; Cerbai, E.; Mugelli, A. (2009-02-12). "Protective effect of darodipine, a calcium antagonist, on rat cardiomyocytes against oxygen radical-mediated injury". British Journal of Pharmacology. 122 (7): 1353–1360. doi:10.1038/sj.bjp.0701525. ISSN 0007-1188. PMC 1565083. PMID 9421282.
- ↑ Hahn, K. A.; Legendre, A. M.; Schuller, H. M. (1997). "Amputation and dexniguldipine as treatment for canine appendicular osteosarcoma". Journal of Cancer Research and Clinical Oncology. 123 (1): 34–38. doi:10.1007/BF01212612. ISSN 0171-5216. PMID 8996538. S2CID 21129242.
- ↑ Tanaka, Hikaru; Shigenobu, Koki (2002). "Efonidipine hydrochloride: a dual blocker of L- and T-type ca(2+) channels". Cardiovascular Drug Reviews. 20 (1): 81–92. doi:10.1111/j.1527-3466.2002.tb00084.x. ISSN 0897-5957. PMID 12070536.
- ↑ Tamargo, J.; López-Sendón, J.; Delpón, E.; González-Morales, M.; de Miguel, E. (1991). "Cardiovascular effects of the new dihydropyridine derivative elgodipine". Arzneimittel-Forschung. 41 (9): 895–900. ISSN 0004-4172. PMID 1796916.
- ↑ PubChem. "Elnadipine". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-01-05.
- ↑ Bansal, Agam B.; Khandelwal, Gaurav (2022), "Felodipine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 31194320, retrieved 2023-01-05
- ↑ Mayhan, W. G.; Heistad, D. D. (1985). "Effect of flordipine on cerebral blood flow". The Journal of Pharmacology and Experimental Therapeutics. 235 (1): 92–97. ISSN 0022-3565. PMID 4045730.
- ↑ Krzemiński, Tadeusz F.; Hudziak, Damian; Sielańczyk, Andrzej W.; Porc, Maurycy; Kedzia, Agnieszka (2008). "Differential effects of furnidipine and its active metabolites in rat isolated working heart". Vascular Pharmacology. 49 (2–3): 91–96. doi:10.1016/j.vph.2008.06.005. ISSN 1537-1891. PMID 18656554.
- ↑ Ishii, Kiyoshi; Matsuo, Hiroshi; Fukaya, Yasuhiro; Tanaka, Sumiyoshi; Sakaki, Hideyuki; Waki, Mitsunori; Araie, Makoto (2003). "Iganidipine, a new water-soluble Ca2+ antagonist: ocular and periocular penetration after instillation". Investigative Ophthalmology & Visual Science. 44 (3): 1169–1177. doi:10.1167/iovs.02-0482. ISSN 0146-0404. PMID 12601046.
- ↑ Schachter, M. (1991). "Isradipine". Journal of Clinical Pharmacy and Therapeutics. 16 (2): 79–91. doi:10.1111/j.1365-2710.1991.tb00288.x. ISSN 0269-4727. PMID 1830320. S2CID 221850085.
- ↑ Tushar, Chopra; Okusa, Mark Douglas (2019-01-01), Ronco, Claudio; Bellomo, Rinaldo; Kellum, John A.; Ricci, Zaccaria (eds.), "Chapter 63 - Aldosterone Antagonists, Amiloride, and Triamterene", Critical Care Nephrology (Third Edition), Philadelphia: Elsevier, pp. 368–373.e1, ISBN 978-0-323-44942-7, retrieved 2023-01-05
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.