 | |
 | |
| Combination of | |
|---|---|
| Estradiol valerate | Estrogen |
| Prasterone enanthate | Androgen; Estrogen; Neurosteroid |
| Clinical data | |
| Trade names | Gynodian Depot, Binodian Depot, Cidodian Depot, Klimax, Supligol NF |
| Other names | EV/DHEA-E; EV/PE; SH-70833-D |
| Routes of administration | Intramuscular injection |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
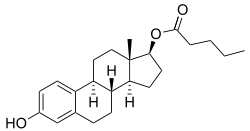
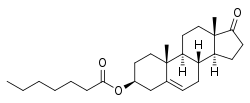
Estradiol valerate/prasterone enanthate (EV/DHEA-E), sold under the brand name Gynodian Depot among others, is an injectable combination medication of estradiol valerate (EV), an estrogen, and prasterone enanthate (DHEA-E), an androgen, estrogen, and neurosteroid, which is used in menopausal hormone therapy for women.[1][2][3][4][5][6][7][8][9] It is provided in the form of 1 mL ampoules containing 4 mg estradiol valerate and 200 mg prasterone enanthate in an oil solution and is administered by intramuscular injection once every 4 to 6 weeks.[2] EV/DHEA-E reportedly has a duration of about 21 days.[10]
The medication is available in Europe, Latin America, and Egypt.[11][12][13][14] EV/DHEA-E was developed and marketed by Schering, was first described in the literature in 1972, and was introduced for medical use in April 1975.[4][15][16][17]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg 1x/1–2 days |
| Methyltestosterone | Metandren, Estratest | Tablet | 0.5–10 mg/day | |
| Fluoxymesterone | Halotestin | Tablet | 1–2.5 mg 1x/1–2 days | |
| Normethandronea | Ginecoside | Tablet | 5 mg/day | |
| Tibolone | Livial | Tablet | 1.25–2.5 mg/day | |
| Prasterone (DHEA)b | – | Tablet | 10–100 mg/day | |
| Sublingual | Methyltestosterone | Metandren | Tablet | 0.25 mg/day |
| Transdermal | Testosterone | Intrinsa | Patch | 150–300 μg/day |
| AndroGel | Gel, cream | 1–10 mg/day | ||
| Vaginal | Prasterone (DHEA) | Intrarosa | Insert | 6.5 mg/day |
| Injection | Testosterone propionatea | Testoviron | Oil solution | 25 mg 1x/1–2 weeks |
| Testosterone enanthate | Delatestryl, Primodian Depot | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone cypionate | Depo-Testosterone, Depo-Testadiol | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone isobutyratea | Femandren M, Folivirin | Aqueous suspension | 25–50 mg 1x/4–6 weeks | |
| Mixed testosterone esters | Climacterona | Oil solution | 150 mg 1x/4–8 weeks | |
| Omnadren, Sustanon | Oil solution | 50–100 mg 1x/4–6 weeks | ||
| Nandrolone decanoate | Deca-Durabolin | Oil solution | 25–50 mg 1x/6–12 weeks | |
| Prasterone enanthatea | Gynodian Depot | Oil solution | 200 mg 1x/4–6 weeks | |
| Implant | Testosterone | Testopel | Pellet | 50–100 mg 1x/3–6 months |
| Notes: Premenopausal women produce about 230 ± 70 μg testosterone per day (6.4 ± 2.0 mg testosterone per 4 weeks), with a range of 130 to 330 μg per day (3.6–9.2 mg per 4 weeks). Footnotes: a = Mostly discontinued or unavailable. b = Over-the-counter. Sources: See template. | ||||
See also
References
- ↑ "Gynodian® Depot" (in German). Zürich: Bayer (Schweiz) AG. May 2017. Archived from the original on 29 May 2019. Retrieved 15 January 2022 – via compendium.ch.
- 1 2 "Gynodian® Depot" (PDF). Bayer Middle East. Archived from the original (PDF) on 8 May 2019 – via HCP MEPPO (Modern Medicine).
- ↑ "Gynodian Depot" (PDF) (in Czech). Berlin: Bayer Pharma AG. 31 October 2011. Archived from the original (PDF) on 29 May 2019. Retrieved 15 January 2022 – via www.sukl.cz.
- 1 2 Picha E, Weghaupt K (March 1972). "[Experience with a new hormone combination for menopausal disorders]" [Experience with a new hormone combination for menopausal disorders]. Medizinische Klinik (in German). 67 (11): 382–386. PMID 4259772.
A new hormone combination for menopausal complaints. Since the treatment of menopausal complaints with estrogens as well as with the combination of estrogens and androgens causes undesired side effects such as bleeding, mammary changes and masculinisation, dehydroepiandrosteron (DHEA), a precursor of testosteron, has been synthesised, which has only a low conversion rate to free testosteron and no masculinising effect. The substance has been tested in combination with estrogen (200 mg DHEA-enanthate and 4 mg estradiolvalerianate per 1 ml) in 266 women with menopausal complaints. The duration of treatment has been up to 6 years with an injection interval of 3 to 8 weeks. The therapeutic results were as good as with estrogen-androgen-combinations, but there was no masculinising effect. Changes of voice, hair and libido caused by pretreatment partly disappeared. Side effects [such] as acne, mastodynia, and sensation of repletion were of transitory nature. This preparation seems to be a true alternative to the traditional estrogen-androgen-combinations.
- ↑ Lauritzen C (1980). "Erfahrungen in der Behandlung klimakterischer Beschwerden mit Depot-Injektionen von Östradiolvalerianat-Dehydroepiandrosteronönanthat" [Experience of treatment of climacteric symptoms with depot injections of estradiol valerianate-dehydroandrosterone enantate]. Die Therapiewoche. 30 (10): 1736–1742. ISSN 0040-5973.
A trial of estradiol valerianate-dehydroandrosterone oenantate (Gynodian-Depot) was conducted in 68 post-menopausal women. The treatment exerted a very favorable influence on the typical subjective disorders of the climacteric and on the atrophic alterations of the target organs. Owing to its estrogenic and dehydroepiandrosterone components, the compound also exerts a favorable psychotropic effect. It was tolerated well and caused no side effects of any significance.
- ↑ Jurczok F (March 1976). "[Treatment of the climacteric symptom complex with a new combined hormone preparation]" [Treatment of the climacteric symptom complex with a new combined hormone preparation]. Fortschritte der Medizin (in German). 94 (9): 524–527. PMID 134967.
- ↑ Dinulović D, Radonjić G (1987). "[Gynodian-depot in the treatment of castration-induced postmenopause]" [Gynodian-depot in the treatment of castration-induced postmenopause]. Jugoslavenska Ginekologija I Perinatologija (in Croatian). 27 (1–2): 37–40. PMID 2960859.
- 1 2 Düsterberg B, Wendt H (1983). "Plasma levels of dehydroepiandrosterone and 17 beta-estradiol after intramuscular administration of Gynodian-Depot in 3 women". Hormone Research. 17 (2): 84–89. doi:10.1159/000179680. PMID 6220949.
- 1 2 Kuhl H, Taubert HD (1987). Das Klimakterium – Pathophysiologie, Klinik, Therapie [The Climacteric – Pathophysiology, Clinic, Therapy] (in German). Stuttgart, Germany: Thieme Verlag. p. 122. ISBN 978-3137008019.
- ↑ Ufer J (1 January 1978). Hormontherapie in der Frauenheilkunde: Grundlagen und Praxis [Hormone Therapy in Gynecology: Principles and Practice] (in German) (5th ed.). de Gruyter. p. 276. ISBN 978-3-11-006664-7. OCLC 924728827.
- ↑ "Prasterone (Dehydroepiandrosterone, DHEA) vaginal Uses, Side Effects & Warnings".
- ↑ Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 2100, 2124–2125. ISBN 978-0-85369-840-1.
- ↑ "Home". micromedexsolutions.com.
- ↑ Muller MF, Dessing RP (19 June 1998). European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. pp. 566–567. ISBN 978-3-7692-2114-5.
- ↑ Sauer F (February 2008). Erfolgsfaktoren für das marktorientierte Management patentgeschützter Arzneimittel: eine Analyse der Produktwahrnehmung niedergelassener Vertragsärzte unter der Berücksichtigung unsicherer Therapieergebnisse. BoD – Books on Demand. pp. 37, 346. ISBN 978-3-936863-12-3.
- ↑ Kaufmann M, Costa SD, Scharl A (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 917–. ISBN 978-3-662-11496-4.
- ↑ Kleemann A, Engel J, Kutscher B, Reichert D (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1172–1174, 2441–2442. ISBN 978-3-13-179525-0.
- ↑ Rauramo L, Punnonen R, Kaihola LH, Grönroos M (January 1980). "Serum oestrone, oestradiol and oestriol concentrations in castrated women during intramuscular oestradiol valerate and oestradiolbenzoate-oestradiolphenylpropionate therapy". Maturitas. 2 (1): 53–58. doi:10.1016/0378-5122(80)90060-2. PMID 7402086.
